��Ŀ����
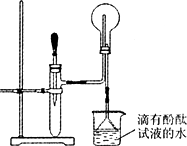
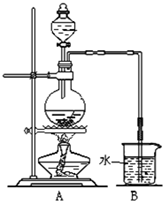
 ��ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ�ã���ʵ��˳���ǣ�
��ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ�ã���ʵ��˳���ǣ��ٰ�ͼ��ʾ��ʵ��װ�����Ӻã�
����U�ι��ڼ���������īˮ����Ʒ�죩��Һ����T�������У�ʹU�ι������ߵ�Һ�洦��ͬһˮƽ�棬�ټн������У�
�����м���Թ���ʢ1g�����ƣ�������2mL���ҵ�����ˮ��ͬʱ�������м��ɹ۲��Իش�
��1��ʵ���й۲쵽��������
U�β�������ĺ�īˮ����Ʒ�죩���ؿ��ڶ�����
U�β�������ĺ�īˮ����Ʒ�죩���ؿ��ڶ�����
����2����ʵ���б�����е�һ��ʵ�������
���װ��������
���װ��������
����3����ʵ���ԭ����
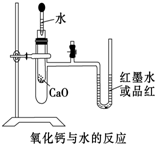
CaO��ˮ��Ӧ�ų�����ʹ���Թ��п������ͣ������īˮ����Ʒ�죩��U�ι��е�Һ�治����ƽ
CaO��ˮ��Ӧ�ų�����ʹ���Թ��п������ͣ������īˮ����Ʒ�죩��U�ι��е�Һ�治����ƽ
����4��˵��CaO��H2O��������Ca��OH��2������֮��Ĺ�ϵ
CaO��H2O�������ʹ���Ca��OH��2������
CaO��H2O�������ʹ���Ca��OH��2������
����5������ʵ����CaO����NaCl��ʵ�黹�ܷ�۲쵽��ͬ����
��
��
����������1�������ƺ�ˮ֮��ķ�Ӧ�Ƿ��ȵģ���������������������ʣ�
��2��������ѹԭ���µ�ʵ������һ��Ҫ��֤װ�ò�©����
��3������ʵ������ҩƷ�������Լ����������������������ش�
��4�����ݷ�Ӧ����������������������֮��Ĵ�С��ϵ������Ӧ�������������
��5���Ȼ��ƺ�ˮ��Ϻ������ı仯�ܲ����ԣ�
��2��������ѹԭ���µ�ʵ������һ��Ҫ��֤װ�ò�©����
��3������ʵ������ҩƷ�������Լ����������������������ش�
��4�����ݷ�Ӧ����������������������֮��Ĵ�С��ϵ������Ӧ�������������
��5���Ȼ��ƺ�ˮ��Ϻ������ı仯�ܲ����ԣ�
����⣺��1�������ƺ�ˮ֮��ķ�Ӧ�Ƿ��ȵģ������Թ����¶����ߣ���������������������ʣ������ڲ�ѹǿ����U�β�������ĺ�īˮ����Ʒ�죩���ؿ��ڶ�������
�ʴ�Ϊ��U�β�������ĺ�īˮ����Ʒ�죩���ؿ��ڶ�������
��2����ʵ����������ѹԭ���µ�ʵ��������֣�����ʵ��֮ǰһ��Ҫ���װ�������ԣ�
�ʴ�Ϊ�����װ�������ԣ�
��3��CaO��ˮ��Ӧ�ų�������ʹ���Թ��п������ͣ��ڲ�ѹǿ���������īˮ����Ʒ�죩��U�ι��е�Һ�治����ƽ��
�ʴ�Ϊ��CaO��ˮ��Ӧ�ų�����ʹ���Թ��п������ͣ������īˮ����Ʒ�죩��U�ι��е�Һ�治����ƽ��
��4��CaO+H2O�TCa��OH��2������ʵ������֪���������ƺ�ˮ֮��ķ�Ӧ�Ƿ��ȵģ�CaO��H2O�������ʹ���Ca��OH��2��������
�ʴ�Ϊ��CaO��H2O�������ʹ���Ca��OH��2��������
��5���Ȼ��ƺ�ˮ��Ϻ������ı仯�ܲ����ԣ��Թ�������ѹǿ�������䣬��������κ�����
�ʴ�Ϊ����
�ʴ�Ϊ��U�β�������ĺ�īˮ����Ʒ�죩���ؿ��ڶ�������
��2����ʵ����������ѹԭ���µ�ʵ��������֣�����ʵ��֮ǰһ��Ҫ���װ�������ԣ�
�ʴ�Ϊ�����װ�������ԣ�
��3��CaO��ˮ��Ӧ�ų�������ʹ���Թ��п������ͣ��ڲ�ѹǿ���������īˮ����Ʒ�죩��U�ι��е�Һ�治����ƽ��
�ʴ�Ϊ��CaO��ˮ��Ӧ�ų�����ʹ���Թ��п������ͣ������īˮ����Ʒ�죩��U�ι��е�Һ�治����ƽ��
��4��CaO+H2O�TCa��OH��2������ʵ������֪���������ƺ�ˮ֮��ķ�Ӧ�Ƿ��ȵģ�CaO��H2O�������ʹ���Ca��OH��2��������
�ʴ�Ϊ��CaO��H2O�������ʹ���Ca��OH��2��������
��5���Ȼ��ƺ�ˮ��Ϻ������ı仯�ܲ����ԣ��Թ�������ѹǿ�������䣬��������κ�����
�ʴ�Ϊ����
����������ͨ��ʵ����ʽ��̽�����ȷ�Ӧ�����Ը�����ѧ֪ʶ���ش�ע��֪ʶ��Ǩ�ƺ�Ӧ���ǹؼ����ѶȲ���

��ϰ��ϵ�д�
 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ
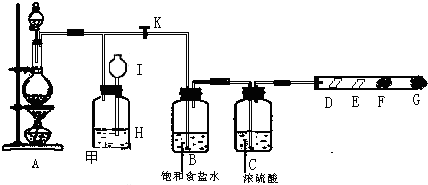
��ͼ��ijͬѧ��Ƶ��Ʊ���������������ϵ��ʵ���װ�ã��гּ������������ԣ���
��1����װ��A���Ʊ�����ѡ�õ�ҩƷΪ����������̺�Ũ���ᣬ��д��װ��A�еĻ�ѧ��Ӧ����ʽ�� ��ʵ����Ҳ���ø��������������2KMnO4+16HCl��Ũ��=5Cl2��+2MnCl2+2KCl+8H2O�÷�Ӧ�е��������� ��������0.2mol���������������Ļ�ԭ�������ʵ����� mol��
��2��װ��B�б���ʳ��ˮ�������� ��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η��� ��
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢�ⵥ�ʵ�������ǿ��������D�л���ͨ����������ʱ�����Կ���D����ɫ��Һ��Ϊ ɫ������Dװ�õĻ�����ʹD����Һ����Eװ�ã�����ƿ����һ��������Թ۲쵽�������� ��
��5��װ��F����������NaOH��Һ�������ȣ���д����Ӧ�����ӷ���ʽ ��

��1����װ��A���Ʊ�����ѡ�õ�ҩƷΪ����������̺�Ũ���ᣬ��д��װ��A�еĻ�ѧ��Ӧ����ʽ��
��2��װ��B�б���ʳ��ˮ��������
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η���
| a | b | c | d | |
| I | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| II | ��ʯ�� | �轺 | ��ʯ�� | ��ˮ�Ȼ��� |
| III | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��5��װ��F����������NaOH��Һ�������ȣ���д����Ӧ�����ӷ���ʽ

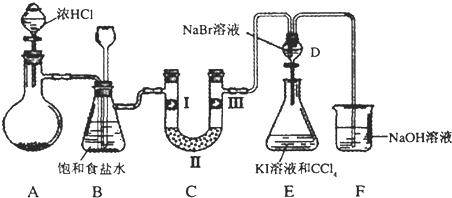
 ��ͼ��ij�о���ѧϰС������ʵ������������װ�������ȡ��ˮ���������ʵ�飮
��ͼ��ij�о���ѧϰС������ʵ������������װ�������ȡ��ˮ���������ʵ�飮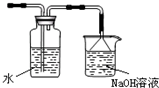 ��
��