��Ŀ����
����Ŀ��������Ԫ��X��Y��Z��M��ԭ������������������X��Y��Z ����Ԫ���У����γɺ�����Ԫ�ص�10������m��n��p��q�����з�Ӧm+n![]() p
p![]() +q��M������������Ӧ��ˮ����Ϊ��ǿ�ᡣ������˵����ȷ��
+q��M������������Ӧ��ˮ����Ϊ��ǿ�ᡣ������˵����ȷ��
A. ԭ�Ӱ뾶X<M<Z<Y B. �ǽ�����X <M<Z<Y
C. X��Y��Z����Ԫ����ɵĻ������ˮ��Һһ�������� D. MZ2����������ˮ��ɱ������
���𰸡�D
��������
������Ԫ��X��Y��Z��M��ԭ������������������X��Y��Z ����Ԫ���У����γɺ�����Ԫ�ص�10������m��n��p��q�����з�Ӧ![]() �����ϴ˷�Ӧ��ӦΪNH4+
�����ϴ˷�Ӧ��ӦΪNH4+![]() OH-
OH-![]() NH3
NH3![]() H2O������XΪH��YΪN��ZΪO��MΪ�����ڣ�����������Ӧ��ˮ����Ϊ��ǿ�ᣬ MΪCl��
H2O������XΪH��YΪN��ZΪO��MΪ�����ڣ�����������Ӧ��ˮ����Ϊ��ǿ�ᣬ MΪCl��
A.������������֪ԭ�Ӱ뾶��СӦΪ��X< Z <Y< M����A����
B.������������֪�ǽ����ԣ�Y <Z����B����
C.X��Y��Z����Ԫ����ɵĻ������ˮ��Һ���ܳ����ԣ����磺HNO3��NH4NO3��Ҳ�����Լ��ԣ�����NH3��H2O����C����
D.������������֪��MZ2��ClO2������ǿ�����ԣ�����������ˮ��ɱ����������D��ȷ��
�����ΪD��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���ƵĻ�������;�㷺���ش��������⣺
��1��������(Na2Sx)�����ۺϵ���ֹ������ԭ�Ӽ۲���ӵĹ������ʽ(�����Ų�ͼ)Ϊ___________����̬Sԭ�ӵ���ռ������ܼ��ĵ���������ͼΪ___________������Na2S4�д��ڵĻ�ѧ���У�___________��
A.���Ӽ� B.���Թ��ۼ� C.���� D.�Ǽ��Թ��ۼ�
��2��r(S2��)>r(Na+)��ԭ����___________��
��3��Na2SO3��������ҵ����������Ư�����������ӵ�����ԭ�ӵ��ӻ���ʽ��___________���ռ乹����______________________��
��4���±��г����Ƶ�±������۵㣺
��ѧʽ | NaF | NaCl | NaBr | NaI |
�۵�/�� | 995 | 801 | 775 | 651 |
��NaF���۵��NaI���۵�ߵ�ԭ����_________________________________��
��NaCl��������786 kJ/mol����NaF�ľ����ܿ�����___________��
A. 704 kJ/mol B. 747kJ/mol C 928 kJ/mol
��5��NaH����NaCl�͵���������ṹ����֪NaH����ľ�������a=488pm��Na+�뾶Ϊ102pm����H���İ뾶Ϊ___________pm��NaH�������ܶ���___________g��cm��3(������λ��Ч����)��[H��1��Na��23]
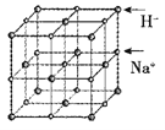
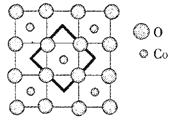
��6�����������ֵĴ��ᾧˮ�ij������Ͼ��廯ѧʽΪNa0.35CoOx��1.3H2O������������CoO2��H2O��Na��H2O��CoO2��H2O��Na��H2O��������״�ṹ����֪CoOx��Ĺ��Ͳ�����ͼ�����д������������ά��������x=___________��