��Ŀ����
���ܱ������н��е����·�Ӧ��2SO2(g)��O2(g) 2SO3(g)��SO2����ʼŨ����0.4 mol��L��1��O2����ʼŨ����1 mol��L��1����SO2��ת����Ϊ80%ʱ����Ӧ�ﵽƽ��״̬��
2SO3(g)��SO2����ʼŨ����0.4 mol��L��1��O2����ʼŨ����1 mol��L��1����SO2��ת����Ϊ80%ʱ����Ӧ�ﵽƽ��״̬��
(1)��Ӧ��ƽ�ⳣ��Ϊ________��
(2)����ƽ��ʱ��Ӧ������ѹǿ����1����ƽ�⽫________(�����������)�ƶ���
(3)��ƽ��ʱ��Ӧ������ѹǿ��С1����ƽ�⽫________(�����������)�ƶ���
(4)ƽ��ʱ����������䣬��ƽ���������г���ϡ������Ar��ʹ��ϵ��ѹ��Ϊԭ����3����ƽ�⽫________(�����������)�ƶ���
 2SO3(g)��SO2����ʼŨ����0.4 mol��L��1��O2����ʼŨ����1 mol��L��1����SO2��ת����Ϊ80%ʱ����Ӧ�ﵽƽ��״̬��
2SO3(g)��SO2����ʼŨ����0.4 mol��L��1��O2����ʼŨ����1 mol��L��1����SO2��ת����Ϊ80%ʱ����Ӧ�ﵽƽ��״̬��(1)��Ӧ��ƽ�ⳣ��Ϊ________��
(2)����ƽ��ʱ��Ӧ������ѹǿ����1����ƽ�⽫________(�����������)�ƶ���
(3)��ƽ��ʱ��Ӧ������ѹǿ��С1����ƽ�⽫________(�����������)�ƶ���
(4)ƽ��ʱ����������䣬��ƽ���������г���ϡ������Ar��ʹ��ϵ��ѹ��Ϊԭ����3����ƽ�⽫________(�����������)�ƶ���
(1)19.0(mol��L��1)��1
(2)����(3)����(4)��
(2)����(3)����(4)��
����������������2SO2(g)��O2(g) 2SO3(g)
2SO3(g)
��ʼŨ��/mol��L��1����0.4��������1��������0
ת��Ũ��/mol��L��1 0.4��80% 0.16 0.32
ƽ��Ũ��/mol��L��1 0.08 0.84 0.32
(1)ƽ�ⳣ��
K��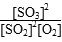 ��
��
��19.0(mol��L��1)��1
(2)ѹǿ����1��������ֵ�Ũ������1����
Q�� ��
�� ��9.5(mol��L��1)��1��19.0(mol��L��1)��1��Q��K��ƽ�������ƶ���
��9.5(mol��L��1)��1��19.0(mol��L��1)��1��Q��K��ƽ�������ƶ���
(3)ѹǿ��С1��������ֵ�Ũ��Ҳ��С1��
Q��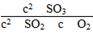 ��
�� ��38.1(mol��L��1)��1��19.0(mol��L��1)��1��Q��K��ƽ�������ƶ���
��38.1(mol��L��1)��1��19.0(mol��L��1)��1��Q��K��ƽ�������ƶ���
(4)����������䣬����ϡ������Ar������ֵ�Ũ�Ȳ��䣬ƽ�ⲻ�ƶ���
 2SO3(g)
2SO3(g)��ʼŨ��/mol��L��1����0.4��������1��������0
ת��Ũ��/mol��L��1 0.4��80% 0.16 0.32
ƽ��Ũ��/mol��L��1 0.08 0.84 0.32
(1)ƽ�ⳣ��
K��
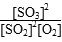 ��
��
��19.0(mol��L��1)��1
(2)ѹǿ����1��������ֵ�Ũ������1����
Q��
 ��
�� ��9.5(mol��L��1)��1��19.0(mol��L��1)��1��Q��K��ƽ�������ƶ���
��9.5(mol��L��1)��1��19.0(mol��L��1)��1��Q��K��ƽ�������ƶ���(3)ѹǿ��С1��������ֵ�Ũ��Ҳ��С1��
Q��
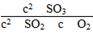 ��
�� ��38.1(mol��L��1)��1��19.0(mol��L��1)��1��Q��K��ƽ�������ƶ���
��38.1(mol��L��1)��1��19.0(mol��L��1)��1��Q��K��ƽ�������ƶ���(4)����������䣬����ϡ������Ar������ֵ�Ũ�Ȳ��䣬ƽ�ⲻ�ƶ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 CO2(g)��Cu(s)�ͷ�Ӧ��H2(g)��CuO(s)
CO2(g)��Cu(s)�ͷ�Ӧ��H2(g)��CuO(s)
 N2(g)��2CO2(g)����H����113.0 kJ/mol���ﵽƽ������¶Ȳ��䣬��С������������´ﵽƽ���H��С
N2(g)��2CO2(g)����H����113.0 kJ/mol���ﵽƽ������¶Ȳ��䣬��С������������´ﵽƽ���H��С CO(g)��H2O����H1
CO(g)��H2O����H1 bY(g)��cZ(g)�ﵽƽ��״̬�����������ѹ����ԭ����1/2���Ҵﵽ�µ�ƽ��״̬ʱ��X�����ʵ���Ũ�ȴ�0.1 mol/L����0.19 mol/L�������ж���ȷ����(����)
bY(g)��cZ(g)�ﵽƽ��״̬�����������ѹ����ԭ����1/2���Ҵﵽ�µ�ƽ��״̬ʱ��X�����ʵ���Ũ�ȴ�0.1 mol/L����0.19 mol/L�������ж���ȷ����(����) 2CO2(g)��N2(g)��ƽ�ⳣ��K��10������������ﵽƽ��״̬����(����)
2CO2(g)��N2(g)��ƽ�ⳣ��K��10������������ﵽƽ��״̬����(����) pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����:
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����: �� ��
�� �� Fe(SCN)2��(aq)����֪ƽ��ʱ�����ʵ���Ũ��c[Fe(SCN)2��]���¶�T�Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���ǣ� ��
Fe(SCN)2��(aq)����֪ƽ��ʱ�����ʵ���Ũ��c[Fe(SCN)2��]���¶�T�Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���ǣ� ��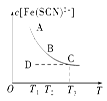
 N2O4(��ɫ)�Ŀ��淴Ӧ��,����״̬һ������ƽ��״̬����(����)��
N2O4(��ɫ)�Ŀ��淴Ӧ��,����״̬һ������ƽ��״̬����(����)��