��Ŀ����
����Ŀ���±���25��ʱ��������ĵ���ƽ�ⳣ����
��ѧʽ | HA��A����ij������� | HNO2 | H2CO3 |
Ka | Ka��4.9��10��10 | Ka��4.6��10��4 | Ka1��4.1��10��7 Ka2��5.6��10��11 |
�ش��������⣺
��1�������ӷ���ʽ��ʾNaNO2��Һ�ʼ��Ե�ԭ��____________________��
��2��A����CO32����HCO3����ˮ�н��H+�������ɴ�С��˳��Ϊ____________��
��3��25��ʱ�������ʵ���Ũ�ȵ�HA��NaA�Ļ����Һ�ʼ��ԣ���û����Һ�и�����Ũ�ȴ�С��ϵΪ________________�����ͻ����Һ�ʼ��Ե�ԭ��_________________________________��
��4��д��NaA��Һ��ͨ����CO2�����ӷ���ʽ_______________________________________��
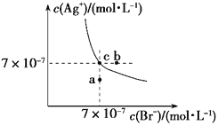
��5��ijͬѧ��Na2CO3��NaHCO3��Һ��������ͼ��ʾʵ����

�� ��ַ�Ӧ��a�Թ��д������ڵ�������_______________________________________��
�� �����ӷ���ʽ��ʾb�Թ��з����ķ�Ӧ_______________________________________��
���𰸡� NO2-+H2O![]() HNO2+OH- CO32-��A-��HCO3- c��Na+����c��A-����c��OH-����c��H+�� HA����̶�С��A��ˮ��̶� A-+CO2+H2O��HA+HCO3- Na+��Cl- 2HCO3-+Ca2+��CaCO3��+CO2��+H2O
HNO2+OH- CO32-��A-��HCO3- c��Na+����c��A-����c��OH-����c��H+�� HA����̶�С��A��ˮ��̶� A-+CO2+H2O��HA+HCO3- Na+��Cl- 2HCO3-+Ca2+��CaCO3��+CO2��+H2O
����������1�������������ˮ����Һ�Լ��ԣ�
��2����ĵ���ƽ�ⳣ��Խ�������Ӧ���������ˮ��̶�ԽС���������Ӧ��������ӽ��������Ũ��ԽС��
��3��25��ʱ�������ʵ���Ũ�ȵ�HA��NaA�Ļ����Һ�ʼ���˵��A-����ˮ��̶ȴ���HA����̶ȣ�
��4������ͼ������֪�����ǿ��˳����HNO2��H2CO3��HA��HCO3-���ݴ���д���ӷ���ʽ��
��5���ټ���CaCl2��Һ��ȫ��Ӧ����̼��ƺ��Ȼ��ƣ�
��̼�����ƺ��Ȼ��Ƶ����ʵ����������̼��Ƴ�����������̼�����ˮ��
��1��NaNO2��Һ�ʼ��Ե�ԭ���������������ˮ����Һ�Լ��ԣ����ӷ���ʽΪ��NO2-+H2O![]() HNO2+OH-��
HNO2+OH-��
��2����ĵ���ƽ�ⳣ��Խ�������Ӧ���������ˮ��̶�ԽС���������Ӧ��������ӽ��������Ũ��ԽС������ͼ������֪�����ǿ��˳����HNO2��H2CO3��HA��HCO3-�����������ˮ��̶ȴ�С˳����CO32-��A-��HCO3-��NO2-������������ӽ������������С˳����CO32-��A-��HCO3-��
��3��25��ʱ�������ʵ���Ũ�ȵ�HA��NaA�Ļ����Һ�ʼ���˵��A-����ˮ��̶ȴ���HA����̶ȣ������Һ�и�����Ũ�ȴ�С��ϵΪ��c��Na+����c��A-����c��OH-����c��H+����
��4������ͼ������֪�����ǿ��˳����HNO2��H2CO3��HA��HCO3-�����NaA��Һ��ͨ����CO2�ķ�Ӧ�����ӷ���ʽΪ��A-+CO2+H2O��HA+HCO3-��
��5���ټ���CaCl2��Һ��̼������Һ�з�Ӧ�����ӷ���ʽ��CO32-+Ca2+��CaCO3������ַ�Ӧ��a���д������ڵ�������Na+��Cl-��
�ڸ���ʵ��������ж�̼�����ƺ��Ȼ��Ƶ����ʵ����������̼��Ƴ�����������̼�����ˮ����Ӧ�����ӷ���ʽΪ��2HCO3-+Ca2+��CaCO3��+CO2��+H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�