��Ŀ����
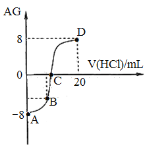
����Ŀ������AG��ʾ��Һ����ȣ������ʽΪ��AG=lg[![]() ]�������£�ʵ��������0.1 mol/L��������Һ�ζ�10 mL 0.1 mol/L MOH��Һ���ζ�������ͼ��ʾ��
]�������£�ʵ��������0.1 mol/L��������Һ�ζ�10 mL 0.1 mol/L MOH��Һ���ζ�������ͼ��ʾ��
����˵���������
A���õζ�����Ӧ��ѡ�������Ϊָʾ��
B��C��ʱ����������Һ�����С��10 mL
C���ζ������д�A�㵽D����Һ��ˮ�ĵ���̶�������
D����B������������Һ���Ϊ5 mL��������Һ�У�c(M+)+2c(H+)=c(MOH)+2c(OH)
���𰸡�C
��������
����������ζ�ǰAG=lg[![]() ]=8������ˮ�����ӻ�������֪������Ũ��Ϊ1011mol/L������������Ũ��Ϊ103 mol/L������MOH�����A��������μӵ������У���ʼʱ��Һ�Լ��ԣ����ζ��ﵽ�յ�ʱ����Һ�ɼ��Ա�Ϊ���ԣ��������Ӿ����ͺ��ԣ��۲���Һ����ɫ��dz����Ƚ���������Ϊ��С�ζ����ڸõζ�������Ӧ��ѡ�������Ϊָʾ������ȷЩ��A��ȷ��B��C��AG=lg[
]=8������ˮ�����ӻ�������֪������Ũ��Ϊ1011mol/L������������Ũ��Ϊ103 mol/L������MOH�����A��������μӵ������У���ʼʱ��Һ�Լ��ԣ����ζ��ﵽ�յ�ʱ����Һ�ɼ��Ա�Ϊ���ԣ��������Ӿ����ͺ��ԣ��۲���Һ����ɫ��dz����Ƚ���������Ϊ��С�ζ����ڸõζ�������Ӧ��ѡ�������Ϊָʾ������ȷЩ��A��ȷ��B��C��AG=lg[![]() ]=0��˵��������Ũ�ȵ���������Ũ�ȣ���Һ�����ԡ�����ǡ�÷�Ӧʱ���ɵ�M+ˮ�⣬��Һ�����ԣ�����C��ʱ����������Һ�����С��10 mL��B��ȷ��C���������ʱˮ�ĵ���̶ȼ�С�����Եζ������д�A�㵽D����Һ��ˮ�ĵ���̶���������С��C����D����B������������Һ���Ϊ5 mL��MOH���������ɵ�MCl��ʣ���MOHŨ����ȣ�����ݵ���غ��֪c(M+)+c(H+)=c(Cl)+c(OH)�����������غ��֪c(M+)+c(MOH)=2c(Cl)�����������Һ�У�c(M+)+2c(H+)=c(MOH)+2c(OH)��D��ȷ����ѡC��
]=0��˵��������Ũ�ȵ���������Ũ�ȣ���Һ�����ԡ�����ǡ�÷�Ӧʱ���ɵ�M+ˮ�⣬��Һ�����ԣ�����C��ʱ����������Һ�����С��10 mL��B��ȷ��C���������ʱˮ�ĵ���̶ȼ�С�����Եζ������д�A�㵽D����Һ��ˮ�ĵ���̶���������С��C����D����B������������Һ���Ϊ5 mL��MOH���������ɵ�MCl��ʣ���MOHŨ����ȣ�����ݵ���غ��֪c(M+)+c(H+)=c(Cl)+c(OH)�����������غ��֪c(M+)+c(MOH)=2c(Cl)�����������Һ�У�c(M+)+2c(H+)=c(MOH)+2c(OH)��D��ȷ����ѡC��

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�