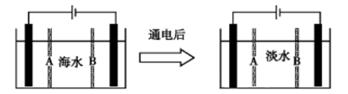
题目内容
已知:
(1)2Fe(s)+O2(g)=2FeO(s)△H=-544kJ?mol-1
(2)2Al(s)+
O2(g)=Al2O3(s)△H=-1675kJ?mol-1
则2Al(s)+3FeO(s)=Al2O3(s)+3Fe(s)的△H为( )
(1)2Fe(s)+O2(g)=2FeO(s)△H=-544kJ?mol-1
(2)2Al(s)+
| 3 |
| 2 |
则2Al(s)+3FeO(s)=Al2O3(s)+3Fe(s)的△H为( )
| A.-2491kJ?mol-1 | B.+859kJ?mol-1 |
| C.-1403kJ?mol-1 | D.-859kJ?mol-1 |
已知:(1)2Fe(s)+O2(g)=2FeO(s)△H=-544kJ?mol-1
(2)2Al(s)+
O2(g)=Al2O3(s)△H=-1675kJ?mol-1
据盖斯定律,(2)-(1)×
得:2Al(s)+3FeO(s)=Al2O3(s)+3Fe(s)△H=-859KJ/mol
故选:D
(2)2Al(s)+
| 3 |
| 2 |
据盖斯定律,(2)-(1)×
| 3 |
| 2 |
故选:D

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目
 NH3(气)+H2S(气),在某一温度下达到平衡,下列各种情况中,不能使平衡发生移动的是
NH3(气)+H2S(气),在某一温度下达到平衡,下列各种情况中,不能使平衡发生移动的是 
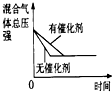
 HCO3-+H+的平衡常数K1=______.(已知:10-5.60=2.5×10-6)
HCO3-+H+的平衡常数K1=______.(已知:10-5.60=2.5×10-6) Sn(s,白)△H3=+2.1kJ?mol-1,
Sn(s,白)△H3=+2.1kJ?mol-1,
 CO2(g)+H2(g) △H<0。在850℃时,平衡常数K=1。
CO2(g)+H2(g) △H<0。在850℃时,平衡常数K=1。