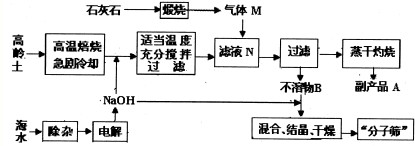
��Ŀ����
��֪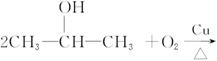
![]()
CH22CH3CH2OH+O2![]() 2CH3CH2CHO+2H2O
2CH3CH2CHO+2H2O
C3H7X+NaOH![]() C3H7OH+NaX
C3H7OH+NaX
�Բο����Ϸ�Ӧ���й����ʵ����ʣ���ʵ��ȷ��ij±����C3H7X������X��λ�ú�����(X������Cl��Br��I����ԭ���е�һ��)��
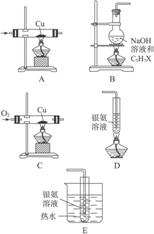
ͼ6-14
(1)�Դ�ͼ6-14��ѡ���ʵ��ļ���ʵ��װ�ò�����һ���ף��Դﵽ����ʵ��Ŀ��(�ñ�Żش��á�����)___________��
(2)��Ӧ��Ϻ���ʢ��������Һ���Թ�����������������C3H7X�Ľṹ��ʽΪ___________����ȡ����B��ƿ�е�Һ�壬��ȴ����ϡ�����ữ���ٵ���___________������������ɫ��������XΪ___________ԭ�ӡ�
(1)B��C��E
(2)CH3CH2CH2X AgNO3��Һ ��
����:
�������Ǹ���Ϣ��Ŀ���ȴ���Ϣ�п�֪��OH��1��̼��2��̼�����ϵĴ����ɱ������������Dz�ͬ�ġ���±�������ʣ�ˮ�����ɴ�����OHλ�ò�ͬ�����������ﲻͬ��ֻ�С�OH��1��̼�ϲ�����ȩ��ֻҪ�������Ƿ���ȩ���ɣ�������B![]() C
C![]() E�Ϳ��ԡ��پ�����������ɫ��������֪��ȩ���ɣ�XΪBr������X����1��̼�ϣ�����֪C3H7X�ĽṹʽΪCH3CH2CH2X��
E�Ϳ��ԡ��پ�����������ɫ��������֪��ȩ���ɣ�XΪBr������X����1��̼�ϣ�����֪C3H7X�ĽṹʽΪCH3CH2CH2X��

��ϰ��ϵ�д�
�����Ŀ