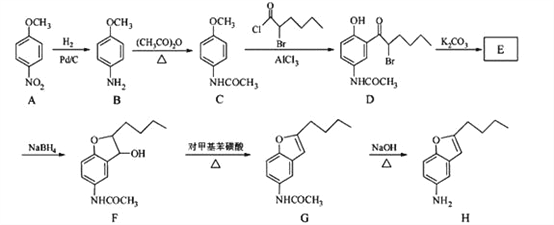
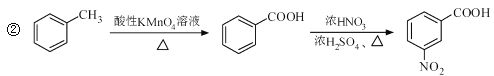
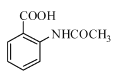
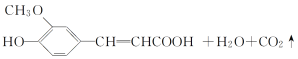��Ŀ����
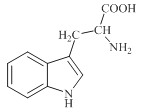
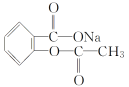
����Ŀ��[��ѧ-ѡ��3�����ʽṹ������]���������й㷺����;���о����֣�������������ṹΪ����(��Li+)�ṩ����Ǩ�Ƶ�ͨ�������С���ȱ�ݡ�������ʹ����е���DZ�������磺ͼ(a)��ʾ��﮳����ӵ���Li3SBF4��ͼ(b)��ʾ���С���ȱ�ݡ���NaCl��

������ѧ֪ʶ�ش��������⣺
(1)�ڱ仯��Cl+e-��Cl-�������У����õ�������ڻ�̬ Cl��________�ܼ����˹��̻�________ (����ա����ͷš�)������
(2)BF4-��B���ӻ���ʽΪ________________����ȵ�����Ϊ___________(��дһ��)������VSEPRģ����ͬ������l�Թµ��ӶԵ���Է���������С�ķ�����___________��
(3)ͼ(a)��ʾ������Li+λ��_____λ�ã�����������BF4-����F-���������������Խ��ͣ�ԭ����______________________________��
(4)ͼ(6)�У���ȱ�ݴ������Na+������__________(��ǡ����ǡ�) NaCl�ľ�������NaCl�����У�Na+�����Cl-�ѻ����ɵ�__________�����϶�С�
(5)������Ϊ���������С���ȱ�ݡ���NaCl���ﵼ��������������Na+Ǩ�Ƶ���һ��λ����ɡ�����Na+����һ����3��Cl-��ɵ���С�����δ���(��ͼc��ʾ)����֪��������a=564 pm��r(Na+)=116pm�� r(Cl-)=167 pm��ͨ�����������δ��װ뾶���жϸ���ʶ�Ƿ���ȷ��__________��(��֪��![]() ��1.414��
��1.414��![]() ��1.732)
��1.732)
���𰸡� 3p �ͷ� sp3 CCl4 NH3 ���� �����������С��ΪLi+�ṩ�Ŀ���Ǩ��ͨ����խ����������Ǩ�� ���� �� ����
�����������������������ʽṹ�⣬���龧���ļ��㣬�ӻ����ͣ��ȵ�����ȣ��Ѷ�һ�㡣
��⣺(1)��Ԫ�غ˵����Ϊ17��Cl�ļ۵����Ų�ʽΪ3s23p5, Cl+e-��Cl-,�õ��ȶ���Cl-,�ͷ�������������õ�������ڻ�̬��3p�ܼ����˹��̻��ͷ�������������ȷ��Ϊ��3p���ͷţ�
(2)BF4-��B�ɼ����Ӷ���Ϊ4���ӻ���ʽΪsp3���ȵ����������ͬ�ļ۵�������ԭ������BF4-�۵�����Ϊ32��ԭ����Ϊ5����ȵ��������ΪCCl4��BF4-��VSEPRģ��Ϊ�����壬����������ռ乹�ͣ�����l�Թµ��ӶԵķ��ӳɼ����Ӷ���Ϊ4-1=3����Է���������С�ķ�����NH3����ˣ�������ȷ��Ϊ��sp3 �� CCl4 �� NH3��
(3)һ��Li3SBF4�����к���3��Li+������Li+λ������λ�ã�12��![]() =3��������������BF4-����F-���������������Խ��ͣ�ԭ���������������С��ΪLi+�ṩ�Ŀ���Ǩ��ͨ����խ����������Ǩ�ơ���ˣ�������ȷ��Ϊ������ �������������С��ΪLi+�ṩ�Ŀ���Ǩ��ͨ����խ����������Ǩ�ƣ�
=3��������������BF4-����F-���������������Խ��ͣ�ԭ���������������С��ΪLi+�ṩ�Ŀ���Ǩ��ͨ����խ����������Ǩ�ơ���ˣ�������ȷ��Ϊ������ �������������С��ΪLi+�ṩ�Ŀ���Ǩ��ͨ����խ����������Ǩ�ƣ�
(4)���Ȼ��ƾ�����Na+�����Cl-�ǵ���������ǰ���������������������ǰ����壬ͼ(6)�У���ȱ�ݴ������Na+����������NaCl��������������NaCl�����У�Na+�����Cl-�ѻ����ɵİ������϶�С���ˣ�������ȷ��Ϊ���������ˣ�
(5) �������δ��װ뾶ΪX,��ͼ��Na+����3��Cl-��ɵ������δ��ڵ���һ��Na+��Ѩ���������δ����ǵȱ������Ρ�D(Cl-��Cl-)��[(a/2)2��(a/2)2]1/2��282![]() =399pm��(rCl-��X)cos30�㣽D(Cl-��Cl-)/2��(rCl-��X)
=399pm��(rCl-��X)cos30�㣽D(Cl-��Cl-)/2��(rCl-��X) ![]() =399�� �����X��63pm������X< r(Na+),����Na+����ͨ�������δ��ף�����ʶ�Ǵ���ġ���ˣ�������ȷ��Ϊ������
=399�� �����X��63pm������X< r(Na+),����Na+����ͨ�������δ��ף�����ʶ�Ǵ���ġ���ˣ�������ȷ��Ϊ������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�