��Ŀ����
�����ʽṹ�����ʡ�
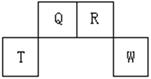
ij��λ������Ϊ����ɫ���壬��ԭ���������������A��B��C��D��E����Ԫ��
��ɣ���ԭ�Ӹ�����Ϊl4:4:5:1:1������C��DԪ��ͬ������ԭ������DΪC��
������EԪ�ص���Χ�����Ų�Ϊ(n-1)dn+6nsl���ش��������⡣
��1��Ԫ��B��C��D�ĵ�һ�����ܵ��ɴ�С����˳��Ϊ ������Ԫ�ط��ű�ʾ��
��2��DԪ��ԭ�ӵ����������Ų�ͼΪ ��
��3������λ������Ļ�ѧʽΪ______�����������ԭ�ӵ��ӻ���ʽΪ ��
��4��CԪ�ؿ���AԪ���γ����ֳ����Ļ������ԭ�Ӹ����ȷֱ�Ϊ1��1��l��2�����ֻ����������Ȼ��ܣ���������Ҫԭ��Ϊ ��
��5��AԪ����BԪ�ؿ��γɷ���ʽΪA2B2��ij������û�����ķ��Ӿ���ƽ��ṹ������ṹʽΪ �������к��� ���Ҽ��� ���м���
��6��AԪ����EԪ�ؿ��γ�һ�ֺ�ɫ������侧��ṹ��Ԫ��ͼ����û�����Ļ�ѧʽΪ ���û��������������ȼ�գ�����һ���ػ�ɫ�����һ�����壬д���÷�Ӧ�Ļ�ѧ����ʽ ��
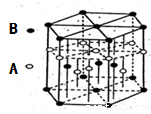
ij��λ������Ϊ����ɫ���壬��ԭ���������������A��B��C��D��E����Ԫ��
��ɣ���ԭ�Ӹ�����Ϊl4:4:5:1:1������C��DԪ��ͬ������ԭ������DΪC��
������EԪ�ص���Χ�����Ų�Ϊ(n-1)dn+6nsl���ش��������⡣
��1��Ԫ��B��C��D�ĵ�һ�����ܵ��ɴ�С����˳��Ϊ ������Ԫ�ط��ű�ʾ��
��2��DԪ��ԭ�ӵ����������Ų�ͼΪ ��
��3������λ������Ļ�ѧʽΪ______�����������ԭ�ӵ��ӻ���ʽΪ ��
��4��CԪ�ؿ���AԪ���γ����ֳ����Ļ������ԭ�Ӹ����ȷֱ�Ϊ1��1��l��2�����ֻ����������Ȼ��ܣ���������Ҫԭ��Ϊ ��
��5��AԪ����BԪ�ؿ��γɷ���ʽΪA2B2��ij������û�����ķ��Ӿ���ƽ��ṹ������ṹʽΪ �������к��� ���Ҽ��� ���м���
��6��AԪ����EԪ�ؿ��γ�һ�ֺ�ɫ������侧��ṹ��Ԫ��ͼ����û�����Ļ�ѧʽΪ ���û��������������ȼ�գ�����һ���ػ�ɫ�����һ�����壬д���÷�Ӧ�Ļ�ѧ����ʽ ��

��1��N>O>S
��2��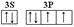
��3��[Cu(NH3)4]SO4?H2O�� sp3�ӻ�
��4��H2O��H2O2֮���γ����
��5��H-N=N-H��3��1
��6��CuH 2CuH+3Cl2 2CuCl2+2HCl
2CuCl2+2HCl
��2��
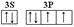
��3��[Cu(NH3)4]SO4?H2O�� sp3�ӻ�
��4��H2O��H2O2֮���γ����
��5��H-N=N-H��3��1
��6��CuH 2CuH+3Cl2
 2CuCl2+2HCl
2CuCl2+2HClij��λ������Ϊ����ɫ���壬��ԭ��������С�����A��B��C��D��E����Ԫ�ع��ɣ���ԭ�Ӹ�����Ϊ14��4��5��1��1������C��DԪ��ͬ������ԭ������DΪC�Ķ�������CΪOԪ�ء�DΪSԪ�أ�EԪ�ص���Χ�����Ų�Ϊ��n-l��dn+6nsl����n+6=10����n=4��������Χ�����Ų�Ϊ3d104sl����EΪCu���ʸ�����ɫ����Ӧ����[Cu(NH3)4]2+��SO42-�����ԭ��������֪AΪH��BΪN����ԭ����Ŀ֮�ȣ���֪������ﺬ��1���ᾧˮ�����仯ѧʽΪ��[Cu(NH3)4]SO4?H2O��
��1��ͬ�������϶��µ�һ�����ܼ�С����OԪ�ص�һ�����ܴ���SԪ�أ�O��NԪ��ͬ���ڣ�NԪ��ԭ��2p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܴ���OԪ�أ��ʵ�һ�������ɴ�С������˳��Ϊ��N��O��S��
��2��DΪSԪ�أ���ԭ�ӵ����������Ų�ͼΪ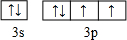 ��
��
��3��������������֪������λ������Ļ�ѧʽΪ��[Cu(NH3)4]SO4?H2O������ΪNH3��Nԭ�Ӽ۲���Ӷ���=3+��5?1��3��/2=4��Nԭ�Ӳ�ȡsp3�ӻ���
��4��OԪ�ؿ���HԪ���γ����ֳ����Ļ������ԭ�Ӹ����ȷֱ�Ϊ1��1��l��2����ΪH2O��H2O2�����ֻ����������Ȼ��ܣ�����Ҫԭ��Ϊ��H2O��H2O2����֮����������
��5��HԪ����NԪ�ؿ��γɷ���ʽΪN2H2�Ļ�����û�����ķ��Ӿ���ƽ��ṹ��Nԭ��֮���γ�N=N˫����Nԭ����Hԭ��֮���γ�N-H������ṹʽΪH-N=N-H�������к���3���Ҽ���1���м���
��6��HԪ����CuԪ�ؿ��γ�һ�ֺ�ɫ������ɾ���ṹ��Ԫ��֪��4��Hԭ��λ���ڲ���6��Hԭ��λ�����ϣ�������Hԭ����Ŀ=4+6��1/3=6��3��Cuԭ��Ϊ�ڲ���2��λ�����ġ�12��λ�ڶ��㣬�ʾ�����Cuԭ����Ŀ=3+2��1/2+12��1/6=6���ʸû�����Ļ�ѧʽΪCuH���û��������������ȼ�գ�����һ���ػ�ɫ�����һ�����壬Ӧ����CuCl2��HCl����Ӧ����ʽΪ��
2CuH+3Cl2 2CuCl2+2HCl��
2CuCl2+2HCl��
��1��ͬ�������϶��µ�һ�����ܼ�С����OԪ�ص�һ�����ܴ���SԪ�أ�O��NԪ��ͬ���ڣ�NԪ��ԭ��2p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܴ���OԪ�أ��ʵ�һ�������ɴ�С������˳��Ϊ��N��O��S��
��2��DΪSԪ�أ���ԭ�ӵ����������Ų�ͼΪ
 ��
����3��������������֪������λ������Ļ�ѧʽΪ��[Cu(NH3)4]SO4?H2O������ΪNH3��Nԭ�Ӽ۲���Ӷ���=3+��5?1��3��/2=4��Nԭ�Ӳ�ȡsp3�ӻ���
��4��OԪ�ؿ���HԪ���γ����ֳ����Ļ������ԭ�Ӹ����ȷֱ�Ϊ1��1��l��2����ΪH2O��H2O2�����ֻ����������Ȼ��ܣ�����Ҫԭ��Ϊ��H2O��H2O2����֮����������
��5��HԪ����NԪ�ؿ��γɷ���ʽΪN2H2�Ļ�����û�����ķ��Ӿ���ƽ��ṹ��Nԭ��֮���γ�N=N˫����Nԭ����Hԭ��֮���γ�N-H������ṹʽΪH-N=N-H�������к���3���Ҽ���1���м���
��6��HԪ����CuԪ�ؿ��γ�һ�ֺ�ɫ������ɾ���ṹ��Ԫ��֪��4��Hԭ��λ���ڲ���6��Hԭ��λ�����ϣ�������Hԭ����Ŀ=4+6��1/3=6��3��Cuԭ��Ϊ�ڲ���2��λ�����ġ�12��λ�ڶ��㣬�ʾ�����Cuԭ����Ŀ=3+2��1/2+12��1/6=6���ʸû�����Ļ�ѧʽΪCuH���û��������������ȼ�գ�����һ���ػ�ɫ�����һ�����壬Ӧ����CuCl2��HCl����Ӧ����ʽΪ��
2CuH+3Cl2
 2CuCl2+2HCl��
2CuCl2+2HCl��
��ϰ��ϵ�д�
�����Ŀ