��Ŀ����
�л��������ǵ���������������أ�����˵������ȷ���ǣ�������
| A���Ҵ��ȿ�����ȼ�ϣ�Ҳ�������ճ�������ʹ�ã�����ˮ�Ҵ���������ɱ�������� | B������һ����Ҫ�Ļ���ԭ�ϣ��ɷ���ȡ����Ӧ��ȡ�ȱ����屽����������л��� | C����֬���ڸ�֬���������������֬����������������� | D����ȩ�������к�������ȩ��ˮ��Һ�����ڱ걾�ķ�������ȩ�������ȩ��֬��ԭ�� |
������A���������Ϊ75%�Ҵ���Һ��������ɱ����������
B�����ݱ������ʣ�
C��������֬���������;��
D�����ݼ�ȩ��Σ������;��
B�����ݱ������ʣ�
C��������֬���������;��
D�����ݼ�ȩ��Σ������;��
����⣺A���Ҵ��ȿ�����ȼ�ϣ�Ҳ�������ճ�������ʹ�ã����������Ϊ75%�Ҵ���Һ��������ɱ������������A����
B������һ����Ҫ�Ļ���ԭ�ϣ����������������塢Ũ���ᷢ��ȡ����Ӧ�ֱ������ȱ����屽����������л����B��ȷ��
C����֬���ڸ�֬���������������֬����������������ᣬ��C��ȷ��
D����ȩ�������к�������ȩ��ˮ��Һ��ʹ�����ʱ��ԣ������ڱ걾�ķ�������ȩ�������ȩ��֬��ԭ�ϣ���D��ȷ��
��ѡ��A��
B������һ����Ҫ�Ļ���ԭ�ϣ����������������塢Ũ���ᷢ��ȡ����Ӧ�ֱ������ȱ����屽����������л����B��ȷ��
C����֬���ڸ�֬���������������֬����������������ᣬ��C��ȷ��
D����ȩ�������к�������ȩ��ˮ��Һ��ʹ�����ʱ��ԣ������ڱ걾�ķ�������ȩ�������ȩ��֬��ԭ�ϣ���D��ȷ��
��ѡ��A��
������������Ҫ���������ʵ���������;���ѶȲ���ע��֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
�л��������ǵ�����������أ�������������Ҫ�ɷֲ����л�����ǣ�������
| A������ | B��ʳ�� | C������ | D��ʳ�� |
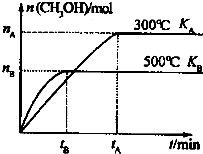 CO��H2�����ǵ�����������ȷ���������أ�
CO��H2�����ǵ�����������ȷ���������أ�