��Ŀ����
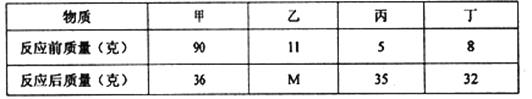
����Ŀ����������(ClNO)�����������ͺϳ�ϴ�Ӽ�����е�Ϊ-5.5�棬��ˮ�⡣ijѧϰС����ʵ����������ͼ��ʾװ���Ʊ�ClNO����֪:HNO2�������������л�ԭ����AgNO2��ˮ�����������ᣬAgNO2+HNO3=AgNO3+HNO2��
�ش���������:
��1������a������Ϊ_____________��װ��B��������____________��
��2��װ��C�г���©����������____________��
��3��ʵ�鿪ʼʱ���ȴ�K1��K2���ر�K3���ٴ�Һ©��������������ϡ���ᣬ���۲쵽C��______ʱ�ر�K1��K2����װ��D����ƿ��ͨ��ǧ�﴿��Cl2����ƿ�г�������ɫ����ʱ���ٴ�K1��K3���Ʊ�ClNO��
��4��װ��D�и���ܵ�������__________________��
��5��ʵ������У���ѧϰС��ͬѧ�þƾ��ƴ�������ȡNO���Ա�ʵ����ɵIJ���Ӱ����____��________��
��6��ClNO��H2O��Ӧ����HNO2��HCl��
�����ʵ��֤��HNO2������________________��(���ṩ���Լ�:1mol/L���ᡢ1mol/L HNO2��Na��NaNO2��Һ����ɫʯ����ֽ����ɫʯ����ֽ)
��Ҫ��֤ClNO��H2O��Ӧ�����Һ�д���Cl-��HNO2�������IJ������輰��ȷ��˳����_____(�����)��
a.���ձ��еμӹ���KI������Һ����Һ����ɫ
b.ȡ1.0mL����ƿ�в�Ʒ���ձ��У�����10.0mLH2O��ַ�Ӧ
c.���ձ��еμ�����KMnO4��Һ����Һ��ɫ��ȥ
d.���ձ��еμ�����AgNO3��Һ���а�ɫ�������ɣ�����ϡ���ᣬ���裬���а�ɫ����
���𰸡� ������ƿ ��ȥNO�е�HNO3��NO2���� ����C��ѹǿ���� ����ɫ��ȫ��ʧ ��ֹˮ������������ƿ����ClNO��Ӧ �¶ȹ������HNO3�ֽ⣨��ӷ��� ����NO�����ٶȹ��죬NO��������Ӧ�������ݳ� ������պȡNaNO2��Һ�����ں�ɫʯ����ֽ�ϣ���ֽ������˵��HNO2������ bdc
��������(1). ������ƿ��NO������ˮ�������ܴ��ڵ�����HNO3��NO2��������ˮ����B������Ϊ����NO�е�HNO3��NO2�����ʣ�
(2).���ر�K1�����ŷ�Ӧ�Ľ��У�C��ѹǿ���ӣ�����©��������Ϊƽ��ϵͳ����ѹǿ������C��ѹǿ����
(3).Ϊ�˵õ��Ƚϴ�����NO����C�к���ɫ��ȫ��ʧʱ�����ٴ���NO2���壻
(4). ��Ϊ��������(ClNO)����ˮ��Ӧˮ�⣬���Ա����ֹ��ˮ��������������ƿ����ClNO��Ӧ����ΪD�����ã�
(5).��������ƿ�����ȣ���ʹ�÷�ӦѸ�٣���������NO���壬��ʹ�϶��NO����δ�μӷ�Ӧ���ݳ��������У�ͬʱ���ϸߵ��¶ȿ���������ֽ��ӷ��������϶��������壻
(6). ����������Ϊ���ᣬ����������ˮ��������ԣ���ʹ�ò�����պȡNaNO2��ҺͿĨ�ں�ɫ��ʯ����ֽ�ϣ�����ֽ��������˵��������Ϊ���
������ҪʹClNO��H2O��Ӧ��ѡ��b����Ϊ�⻯��Ϊ�����������Ը�������ܽ�������������������Ҫ����֤�����ӵĴ��ڣ�ѡ��d���������������������ӣ����Խ���ʹ�����Ը��������֤�����ᣬѡ��c�������Ϊbdc��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�������й�ʵ���������Ҫ������ע�����Ӧ�þ�������ȷ���ǣ� ��

![]()
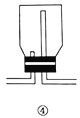
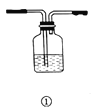
ѡ�� | ʵ����� | ��Ҫ���� | ע������ | Ӧ�þ��� |
A | ϴ�� | �� | �ӳ��ܽ������̹ܳ��� | �ñ���̼��������Һ��ȥCO2�����е�SO2���� |
B | ������� | �� | �Ӵֹܽ�����ϸ�ܳ��� | ����ˮ�Ȼ�������������ﰱ�� |
C | ��Һ | �� | �Ƚ��²�Һ����¿ڷų�,�ٽ��ϲ�Һ����¿ڷų� | ����ֲ���ͺ�ˮ�Ļ���� |
D | �����ռ� | �� | �ӳ��ܽ������̹ܳ��� | �ռ����� |
A. A B. B C. C D. D