��Ŀ����
ͭ�������������������ϵ���У���ش�����������⡣
��1����Ԫ�����ڱ��У�ͭ��������Ԫ��λ��ͬһ�壬���ǻ�̬ԭ�ӵļ۲�����Ų�ʽ�и��ܼ��ϵĵ�������ȣ����ܲ�������������ͨʽΪ________����n��ʾ������Ӳ�������ͭԪ�����������У���̬ԭ��δ�ɶԵ���������ԭ��M �ļ۲���ӹ������ʽΪ___________��
��2����ȩ����Ҫ�Ļ���ԭ�ϣ��� CH3CH2OH ��CH3CHO�ķе�ֱ�Ϊ78.5�桢20.8�棬���ǵ���Է����������2�����е����Ƚϴ�����Ҫԭ��__________��
�� ��H��C��N��O�У���һ����������Ԫ�غ͵縺������Ԫ����ɵĻ�����Ļ�ѧʽΪ_____����һ�ּ��ɣ���CH4��NH3��H2O���ӵļ��ǴӴ�С��˳��Ϊ__________��
��3�����������ᣬ�����ڡ���ˮ�����������·�Ӧ��Au + 4HCl+ HNO3 = H [ AuCl4]+NO + 2H2O����������ˮ����Ҫԭ�����γ���[ AuCl4]-������˽�Ļ����ԡ���[ AuCl4]-����λ������ĿΪ______��д�������ӵĽṹʽ��_____________��
��4��������һ�ֺϽ���н�ǿ�Ĵ����������úϽ�ľ���Ϊ���������ṹ����ԭ��λ�����ģ���ԭ��λ�ڶ��㡣�úϽ�Ļ�ѧʽ���Ա�ʾΪ__________��
��5��Cu(OH)2����������������Һ������������Ũ��ˮ�������ӷ���ʽ��ʾ����Ҫԭ��____________��
 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�

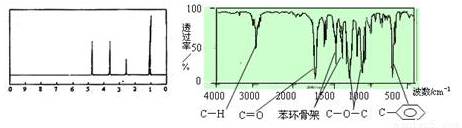
 ���ӽṹ����������ȷ���ǣ�������
���ӽṹ����������ȷ���ǣ������� ��Fe2��֮���γɵĻ�ѧ�����������Ӽ�
��Fe2��֮���γɵĻ�ѧ�����������Ӽ�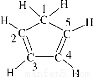 �������н���1��̼ԭ�Ӳ�ȡsp3�ӻ�
�������н���1��̼ԭ�Ӳ�ȡsp3�ӻ�