��Ŀ����
����Ŀ��ϩ������һ����ɫ�д̼�����ζ��Һ��������Ҫ���л��ϳ�ԭ������ṹ��ʽΪ CH2=CH��CH2OH����ش�
��1��ϩ�����г�̼̼˫������еĹ������� �����ƣ���
��2��0.1mol ϩ���������������Ʒ�Ӧ������������ L����״���£���
��3��д��ϩ��������ˮ��Ӧ�Ļ�ѧ����ʽ ��
��4��ϩ������CH3CO18OH����������Ӧ�Ļ�����ʽΪ ��������Ӧ���ɵIJ�����һ�������¿��Է����Ӿ۷�Ӧ�õ��߷��ӻ�������ṹ��ʽΪ ��
��5��ϩ������һ��ͬ���칹���ܷ���������Ӧ�����ṹ��ʽΪ ��
���𰸡����ǻ� ��1.12L
��CH2=CHCH2OH+Br2��CH2BrCH2BrCH2OH
��CH2=CHCH2OH+CH3COOH��CH3COOCH2CH=CH2 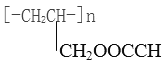
��CH3CH2CHO
��������
�����������ϩ��������̼̼˫�����ǻ��� ��0.1Ħ��ϩ�����ͽ����Ʒ�Ӧ����0.05Ħ������������µ����Ϊ1.12L����ϩ��������ˮ�����ӳɷ�Ӧ������ʽΪ��CH2=CHCH2OH+Br2��CH2BrCH2BrCH2OH
��ϩ���������ǻ����������ᷢ��������Ӧ��������ˮ������ʽΪ��CH2=CHCH2OH+CH3COOH��CH3COOCH2CH=CH2 �����Ľṹ�к���̼̼˫�����ܷ����Ӿ۷�Ӧ����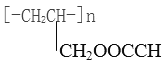
��ϩ������ͬ���칹�к���ȩ���ĽṹΪCH3CH2CHO
