��Ŀ����
 ��֪��25��C��1.013��105Pa�£�1mol������ȫȼ������Һ̬ˮ�ų�285kJ����������ش��������⣺
��֪��25��C��1.013��105Pa�£�1mol������ȫȼ������Һ̬ˮ�ų�285kJ����������ش��������⣺��1����2mol������ȫȼ������ˮ��������ų�������
��
��
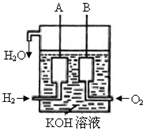
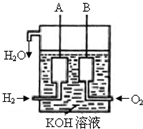
�����������������=����570kJ��2����������������ɴ���ʹ����һ������װ�ã��乹������ͼ��ʾ��A��B�����缫���ɶ��̼����ɣ��õ�ص�����Ϊ��
B
B
����A��B�������õ�ع���ʱ������1mol H2O����·��ת�Ƶ��ӵ����ʵ���Ϊ
2
2
mol����3�����������װ����ͨ���H2�ij�CH4���壬Ҳ�������һ��ԭ���װ�ã���ص��ܷ�Ӧ����ʽΪ��CH4+2O2+2KOH=K2CO3+3H2O����õ�صĸ�����ӦʽΪ��
CH4+8e-+10OH-=CO32-+7H2O
CH4+8e-+10OH-=CO32-+7H2O
����������1������������ȫȼ������Һ̬ˮ��������̬ˮʱ�������仯���ش�
��2��ȼ�ϵ���У�������ͨ��һ�������������ݵ��ת�Ƶ�����������ˮ���������㣻
��3��ȼ�ϵ�صĸ���һ����ȼ��ʧ���ӵĹ��̣�
��2��ȼ�ϵ���У�������ͨ��һ�������������ݵ��ת�Ƶ�����������ˮ���������㣻
��3��ȼ�ϵ�صĸ���һ����ȼ��ʧ���ӵĹ��̣�
����⣺��1��1mol������ȫȼ������Һ̬ˮ�ų�285kJ������������2mol������ȫȼ������Һ̬ˮ�ų�570kJ������������Һ̬ˮ��Ϊ��̬ˮʱ��Ҫ����һ��������������2mol������ȫȼ������ˮ��������ų���������570kJ���ʴ�Ϊ������
��2��ȼ�ϵ���У�������ͨ��һ��������������B�������������ݵ�ط�Ӧ��2H2+O2
2H2O��������2molˮʱ��ת�Ƶ���Ϊ4mol���������õ�ع���ʱ������1mol H2O����·��ת�Ƶ��ӵ����ʵ���Ϊ2mol���ʴ�Ϊ��B��2��
��3��ȼ�ϵ�صĸ���һ����ȼ��ʧ���ӵĹ��̣��ڼ��Ի����£�����ʧ���ӵĵ缫��ӦΪ��CH4+8e-+10OH-=CO32-+7H2O���ʴ�Ϊ��CH4+8e-+10OH-=CO32-+7H2O��
��2��ȼ�ϵ���У�������ͨ��һ��������������B�������������ݵ�ط�Ӧ��2H2+O2
| ||
��3��ȼ�ϵ�صĸ���һ����ȼ��ʧ���ӵĹ��̣��ڼ��Ի����£�����ʧ���ӵĵ缫��ӦΪ��CH4+8e-+10OH-=CO32-+7H2O���ʴ�Ϊ��CH4+8e-+10OH-=CO32-+7H2O��
���������⿼��ѧ��ȼ�ϵ�ص����֪ʶ�����Ը�����ѧ�������ش��ѶȲ���

��ϰ��ϵ�д�
 ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�����Ŀ
 I����������20mL0.1mol?L-1Na2CO3��Һ����μ���0.1mol?L-1 HCl��Һ40mL����Һ��pH���ͣ���ʱ��Һ�к�̼Ԫ�ص������ʵ���Ũ�ȵİٷֺ��������ᣩҲ�����仯��CO2���ݳ�δ����������ͼ��ʾ���ش��������⣺
I����������20mL0.1mol?L-1Na2CO3��Һ����μ���0.1mol?L-1 HCl��Һ40mL����Һ��pH���ͣ���ʱ��Һ�к�̼Ԫ�ص������ʵ���Ũ�ȵİٷֺ��������ᣩҲ�����仯��CO2���ݳ�δ����������ͼ��ʾ���ش��������⣺ H++HA-����HA-
H++HA-����HA- H++A2-
H++A2-