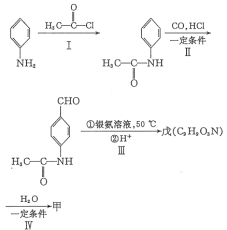
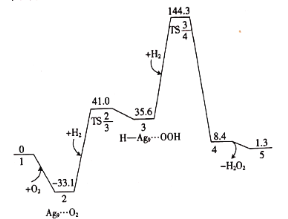
��Ŀ����
����Ŀ��NH4HCO3�ķֽ��¶��� 35�档���Ȼ��غ���ȡ�������ѵĸ���Ʒ��������Ϊԭ�������������Ϻ�������淋ȣ�ԭ�ϵ��ۺ������ʽϸߡ�����Ҫ�������£�
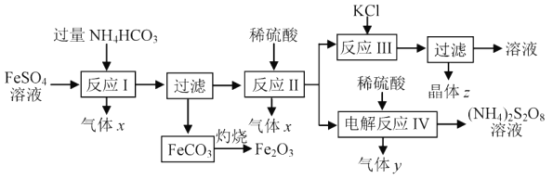
��1������x��_________����Ӧ I ����Ʒ�Ӧ�¶ȵ���35�� , ��Ŀ����_______��
��2����ӦI�����ӷ���ʽΪ ___________��FeCO3���յķ�Ӧ����ʽΪ __________��
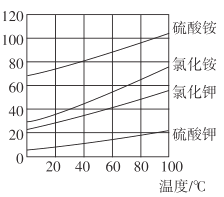
��3�������ʵ��ܽ��������ͼ������z��____��������ӦIII������ԭ�� ________����ҵ�����ϳ��ڷ�ӦIII�Ĺ����м���һ�������Ҵ�����Ŀ����_____________��
��4����ӦIV ����������(NH4)2S2O8 (��������泥������ʱ���ö��Ե缫������y��__________�����������ĵ缫��Ӧ�ɱ�ʾΪ_______________ ��
���𰸡�CO2�������̼ ��ֹ NH4HCO3�ֽ⣨����� Fe2����ˮ�⣩ Fe2++2HCO3- =FeCO3��+H2O+CO2�� 4FeCO3+O2![]() 2Fe2O3+4CO2 K2SO4 ����ͬ�¶��£�K2SO4���ȴﵽ���ͻ� K2SO4�ܽ����С�� K2SO4�ܽ�ȱ� KCl��(NH4)2SO4С�����ӷ�Ӧ�������ܽ�ȸ�С�� K2SO4�ķ�����У��������� ���� K2SO4 ���ܽ�� H2 2SO42��-2e- = S2O82���� 2HSO4��-2e- = 2H++S2O82��
2Fe2O3+4CO2 K2SO4 ����ͬ�¶��£�K2SO4���ȴﵽ���ͻ� K2SO4�ܽ����С�� K2SO4�ܽ�ȱ� KCl��(NH4)2SO4С�����ӷ�Ӧ�������ܽ�ȸ�С�� K2SO4�ķ�����У��������� ���� K2SO4 ���ܽ�� H2 2SO42��-2e- = S2O82���� 2HSO4��-2e- = 2H++S2O82��
��������
��ӦI��FeSO4��NH4HCO3��Ӧ����FeCO3��H2O��CO2�����˵õ�����̼��������̼�����������������������������Ͷ�����̼����ҺΪ����狀�NH4HCO3������Һ�м���ϡ���ᷴӦ��������泥�����識���KCl�������ֽⷴӦ��������غ��Ȼ�泥��������淋õ���������李�
�Ÿ��ݷ����õ�����x��CO2������������ϢNH4HCO3�ķֽ��¶��� 35������˷�ӦI����Ʒ�Ӧ�¶ȵ���35������Ŀ���Ƿ�ֹNH4HCO3�ֽ�(����� Fe2����ˮ��)���ʴ�Ϊ��CO2�������̼����ֹNH4HCO3�ֽ�(����� Fe2����ˮ��)��
�Ʒ�ӦI�� Fe2+��HCO3�� ��Ӧ����FeCO3��H2O��CO2�������ӷ���ʽΪFe2++2HCO3�� = FeCO3��+H2O+CO2����FeCO3������������Ӧ�����������Ͷ�����̼���䷴Ӧ����ʽΪ FeCO3+O2 ![]() 2Fe2O3+4CO2���ʴ�Ϊ��Fe2++2HCO3�� = FeCO3��+H2O+CO2����FeCO3+O2
2Fe2O3+4CO2���ʴ�Ϊ��Fe2++2HCO3�� = FeCO3��+H2O+CO2����FeCO3+O2 ![]() 2Fe2O3+4CO2��
2Fe2O3+4CO2��
�Ǹ����ʵ��ܽ��������ͼ������ͬ�¶��£�K2SO4�ܽ����С�����ӷ�Ӧ�������ܽ�ȸ�С��K2SO4�ķ�����У��������������z��K2SO4����ҵ�����ϳ��ڷ�ӦIII�Ĺ����м���һ�������Ҵ�����Ŀ���ǽ��� K2SO4 ���ܽ�ȣ��ʴ�Ϊ��K2SO4������ͬ�¶��£�K2SO4���ȴﵽ���ͻ� K2SO4�ܽ����С�� K2SO4�ܽ�ȱ� KCl��(NH4)2SO4С�����ӷ�Ӧ�������ܽ�ȸ�С�� K2SO4�ķ�����У��������������� K2SO4���ܽ�ȡ�
�ȷ�ӦIV����������(NH4)2S2O8(���������)����ӦIV�ǵ��������������������(NH4)2S2O8�����ϼ����ߣ�����������Ӧ����������Ӧ����������������ӵõ���������������������y��H2�����������ĵ缫��Ӧ�ɱ�ʾΪ2SO422e�� = S2O82��2HSO4��2e�� = 2H++S2O82���ʴ�Ϊ��H2��2SO422e�� = S2O82��2HSO4��2e�� = 2H++S2O82��

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�����Ŀ��̼���ס����Ԫ���γɵĵ��ʺͻ��������������������Ҫ����;��
(1)���е�ԭ�ӵĵ����Ų�ͼ��ʾ��״̬�У������ɵ͵��ߵ�˳����____(����ĸ)��
A. ![]()
B. ![]()
C. ![]()
D. ![]()
(2)P4S3����������������ӽṹ��ͼ��ʾ��
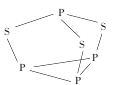
��P4S3��������ԭ�ӵ��ӻ��������Ϊ____��
��ÿ��P4S3�����к��еŵ��ӶԵ���ĿΪ____�ԡ�
(3)��ѧ�Һϳ���һ�������ӡ�N5n+������ṹ�ǶԳƵģ�5��N�ųɡ�V���Σ�ÿ��N���ﵽ8�����ȶ��ṹ���Һ���2�������������˺��ֺϳ���һ�ֺ��С�N5n+���Ļ�ѧʽΪ��N8�������Ӿ���(�þ�����ÿ��Nԭ�Ӷ��ﵽ��8�����ȶ��ṹ)��N8�ĵ���ʽΪ____��(CN)2�м����֮��ļн�Ϊ180�㣬���жԳ��ԣ�������ÿ��ԭ�ӵ�����������8�����ȶ��ṹ����ṹʽΪ____��
(4)ֱ�������������������������������������������ͨ�����ö�����ԭ�����������ģ���ṹ��ͼ��ʾ������n�������������γɵ�������������ӵ�ͨʽΪ____��
(5)̼�����е������Ӳ�ͬ���ȷֽ��¶ȾͲ�ͬ���±�Ϊ����̼���ε��ȷֽ��¶ȺͶ�Ӧ���������ӵİ뾶�����Ž��������Ӱ뾶������̼���ε��ȷֽ��¶������ߣ�ԭ���� ___��
̼���� | MgCO3 | CaCO3 | SrCO3 | BaCO3 |
�ȷֽ��¶�/�� | 402 | 900 | 1172 | 1360 |
���������Ӱ뾶/pm | 66 | 99 | 112 | 135 |
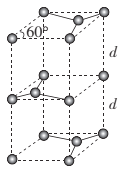
(6)ʯī�ľ����ṹ��ͼ��ʾ����֪ʯī���ܶ�Ϊ��g.cm-3��C-C���ļ���Ϊr cm��MΪ�����ӵ�������ֵ����ʯī����IJ���d= ___cm��
����Ŀ����Ȼ��ˮ�����������ǹ�ҵ��������������Ҫ��������Ӧ�� 400�����Ͻ��С�l 00kPa ʱ���ڷ�Ӧ������ͨ�������Ϊˮ���������Ϊ1 : 5�Ļ�����壬�����±���Ӧ��
��Ӧ����ʽ | �ʱ��H(kJ/mol) | 600��ʱ��ƽ�ⳣ�� |
��CH4(g)+ H2O(g) | a | 0.6 |
��CH4(g)+ 2H2O(g) | +165.0 | b |
��CO(g)+ H2O(g) | -41.2 | 2.2 |
��ش������������⣺
��1���ϱ������� a=__________; b= ___________ ��
��2�����ڷ�Ӧ�ڣ����ܼӿ췴Ӧ�������CH4ת���ʵĴ�ʩ��_____________��
A.���� B.�Ӵ��� C.��ѹ D.����CO2
��3�����������˵�������и���Ӧ���ﵽƽ�����___________��
A.��ϵ��H2O��CH4���ʵ���֮�Ȳ��ٱ仯
B.��ϵ��H2������������ֲ���
C.����n ��CO2��ͬʱ����2n��H2O
D. v��(CO)= v��(H2)
��4����ҵ���������У���Ӧ����̼��Ӱ���Ч�ʣ���Ҫ�����¶ȹ����Լ��ٻ�̼������ϵ�в���̼�ķ�Ӧ����ʽΪ _______________��
��5��ƽ��ʱ���£�CO������_________��ѡ�����С��)��
��6��һ���¶��� ��ƽ��ʱ�����ϵ�� CO2��H2�����ʵ���Ũ�ȷֱ���0.75mo l/L��4.80mol/L , ���ʱ��ϵ��CO ���ʵ���Ũ����_______ mol/L��
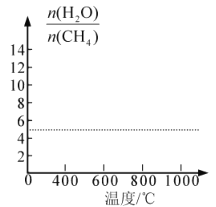
��7���ı�����ƽ����ϵ���¶ȣ�ƽ��ʱH2O��CH4���ʵ���֮��[![]() ��ֵҲ�����Ÿı䣬��ͼ�л�����仯���ơ�________________
��ֵҲ�����Ÿı䣬��ͼ�л�����仯���ơ�________________