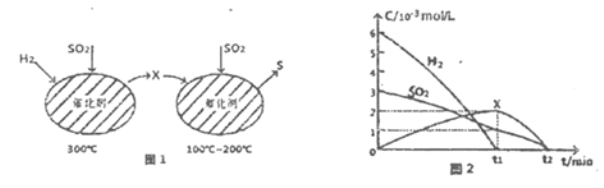
��Ŀ����
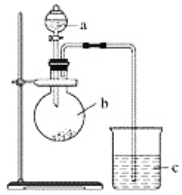
����Ŀ����������(ClNO)�����������ͺϳ�ϴ�Ӽ�����е�Ϊ-5.5�棬��ˮ������HNO2��HCl��ijѧϰС����ʵ����������ͼ��ʾװ���Ʊ�ClNO����֪: HNO2�������������л�ԭ�ԣ�AgNO2����ˮ�����������ᣬAgNO2+HNO3=AgNO3+HNO2��
�ش���������
��1������a������Ϊ_____________�� a��װ��C�е�������____________��
��2��װ��B��������____________��
��3��ʵ�鿪ʼʱ���ȴ�K1��K2���ر�K3���ٴ�Һ©��������������ϡ���ᣬ���۲쵽C��______ʱ�ر�K1��K2����װ��D����ƿ��ͨ����﴿��Cl2����ƿ�г�������ɫ����ʱ���ٴ�K1��K3���Ʊ�ClNO��
��4��װ��D�и���ܵ�������__________________��
��5��ʵ������У���ѧϰС��ͬѧ�þƾ��ƴ�������ȡNO���Ա�ʵ����ɵIJ���Ӱ������з�Ӧ���ʹ��죬ʹNO��������Ӧ�������ݳ��⣬�������У�___________��
��6����Ҫ��֤ClNO��H2O��Ӧ�����Һ�д���Cl-��HNO2�������IJ������輰��ȷ��˳����_____(�����)��
a.���ձ��еμӹ���KI������Һ����Һ����ɫ
b.ȡ1.0mL ����ƿ�в�Ʒ���ձ��У�����10.0mLH2O��ַ�Ӧ
c.���ձ��еμ�����KMnO4��Һ����Һ��ɫ��ȥ
d.���ձ��еμ�����AgNO3��Һ���а�ɫ�������ɣ�����ϡ���ᣬ���裬���а�ɫ����
��������ˮ�Ĺ����лᷴӦ����ClNO����д���÷�Ӧ�Ļ�ѧ����ʽ��__________________��
���𰸡� ����©�� ƽ����ѹ������C��ѹǿ���� ��ȥNO�е�HNO3��NO2���� ����ɫ��ȫ��ʧ ��ֹˮ������������ƿ����ClNO��Ӧ �¶ȹ������HNO3�ֽ⣨��ӷ��� bdc HNO3+ 3HCl== 2H2O + Cl2+ ClNO
��������ʵ�鿪ʼʱ���ȴ�K1��K2���ر�K3����Һ©��������������ϡ���ᣬ��C�к���ɫ��ȫ��ʧ�ر�K1��K2��װ��A��ϡ�����ͭ��Ӧ����NO���壬ͨ��װ��B��ˮ��ȥ�ӷ������ἰNO�Ϳ�����������Ӧ���ɵĶ���������ʹ��NO����װ��C����ʱװ��C������Ϊ����A�в�����NO���壬��Dװ����ͨ����﴿����Cl2����D���г�������ɫ����ʱ����K1��K2����D���Ʊ�ClNO��װ����ˮCaCl2�ĸ����������Ϊ��ֹˮ��������D�У�ʹNOC1ˮ�⣬��Ӧ���ٺ��K3������β����������ֹ��Ⱦ������
(1)����aΪ����©�������ر�K1�����ŷ�Ӧ�Ľ��У�C��ѹǿ���ӣ�����©��������Ϊƽ��ϵͳ����ѹǿ������C��ѹǿ���ʴ�Ϊ������©����ƽ����ѹ������C��ѹǿ����
(2)NO������ˮ�������ܴ��ڵ�����HNO3��NO2��������ˮ����B������Ϊ��ȥNO�е�HNO3��NO2�����ʣ��ʴ�Ϊ����ȥNO�е�HNO3��NO2���壻
(3)Ϊ�˵õ��Ƚϴ�����NO����C�к���ɫ��ȫ��ʧʱ�����ٴ���NO2���壬�ʴ�Ϊ������ɫ��ȫ��ʧ��
(4)��Ϊ��������(ClNO)����ˮ��Ӧˮ�⣬���Ա����ֹ��ˮ��������������ƿ����ClNO��Ӧ��װ��D�и���ܿ��Է�ֹˮ������������ƿ����ClNO��Ӧ���ʴ�Ϊ����ֹˮ������������ƿ����ClNO��Ӧ��
(5)��������ƿ�����ȣ���ʹ�÷�ӦѸ�٣���������NO���壬��ʹ�϶��NO����δ�μӷ�Ӧ���ݳ��������У�ͬʱ���ϸߵ��¶ȿ���������ֽ��ӷ��������϶��������壬�ʴ�Ϊ���¶ȹ������HNO3�ֽ�(��ӷ�)��
(6)������ҪʹClNO��H2O��Ӧ��ѡ��b����Ϊ�⻯��Ϊ�����������Ը�������ܽ�������������������Ҫ����֤�����ӵĴ��ڣ�ѡ��d���������������������ӣ����Խ���ʹ�����Ը��������֤�����ᣬѡ��c���ʴ�Ϊ��bdc��
��������ˮ�Ĺ����лᷴӦ����ClNO�������������ᷴӦ������ClNO��ClNO��ClԪ��Ϊ-1�ۣ�NԪ��Ϊ+3�ۣ����ݻ��ϼ������غ㣬���������ɣ���Ӧ�Ļ�ѧ����ʽΪHNO3+ 3HCl== 2H2O + Cl2+ ClNO���ʴ�Ϊ��HNO3+ 3HCl== 2H2O + Cl2+ ClNO��

����Ŀ�������ǻ������������ֺ������绢������������������Ϊ���Ͻ��ά���������ӷϷ�(��Ҫ�ɷ�ΪV2O5��Fe2O3��SiO2��)�л���V2O5��һ�ֹ�����������ͼ��ʾ: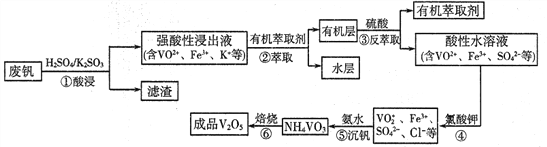
��֪:�����������еı仯���̿ɼ�Ϊ:Rn+(ˮ��)+nHA(�л���)![]() RAn(�л���)+nH+(ˮ��)(ʽ��Rn+��ʾVO2+��Fe3+��HA��ʾ�л���ȡ��)��
RAn(�л���)+nH+(ˮ��)(ʽ��Rn+��ʾVO2+��Fe3+��HA��ʾ�л���ȡ��)��
�ش���������:
��1����������������з���������ԭ��Ӧ�Ļ�ѧ����ʽΪ______________��
��2����ȡʱӦ�����������������___________________��
��3���������з�Ӧ�����ӷ���ʽΪ___________________��
��4��������25��Cʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
ͨ���������ݷ�������ʵ�������У����м��백ˮ��������Һ�����pH��ΧΪ______��
�����백ˮ������ҺpH=2���������ʴﵽ93%�Ҳ�����Fe(OH)3���������ʱ��Һ��c(Fe3+)<_____mol/L(��25����㣬25��ʱKsp[Fe(OH)3]=2.6��10-39)��
��5��V2O5�������������ǿ������Һ����VO2+��ʽ���ڣ�VO2+����ǿ�����ԣ��ܽ�I-����ΪI2����������ԭΪVO+����V2O5������ᷴӦ�����ӷ���ʽΪ_________________��
��6��Ϊ��߷��Ļ����ʣ��������������ν��У��������������ǿ���Խ���Һ����c(VO2+)=amol/L������������ÿ����һ�Σ�VO2+��ȡ��Ϊ80%��4�β�������ǿ���Խ���Һ����c(VO2+)=_______mol/L(��ȡ��=![]() )
)