��Ŀ����
��ͼ��A��B��C��D��E��F��G��Ϊ�л������
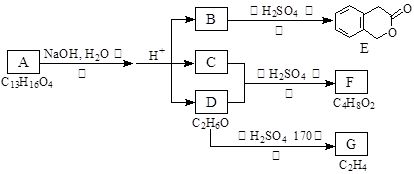
������ͼ�ش����⣺
��1��D�Ļ�ѧ������ ��
��2����Ӧ�۵Ļ�ѧ����ʽ�� �����л������ýṹ��ʽ��ʾ��
��3��B�ķ���ʽ�� ��A�Ľṹ��ʽ�� ����Ӧ�ٵķ�Ӧ������ ��
��4����������3��������B��ͬ���칹�����Ŀ�� ����
�ٺ����ڶ�ȡ�������ṹ������B����ͬ�����š��۲���FeCl3��Һ������ɫ��Ӧ��
д����������һ��ͬ���칹��Ľṹ��ʽ ��
��5��G����Ҫ�Ĺ�ҵԭ�ϣ��û�ѧ����ʽ��ʾG��һ����Ҫ�Ĺ�ҵ��; ��
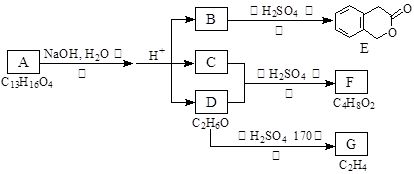
������ͼ�ش����⣺
��1��D�Ļ�ѧ������ ��
��2����Ӧ�۵Ļ�ѧ����ʽ�� �����л������ýṹ��ʽ��ʾ��
��3��B�ķ���ʽ�� ��A�Ľṹ��ʽ�� ����Ӧ�ٵķ�Ӧ������ ��
��4����������3��������B��ͬ���칹�����Ŀ�� ����
�ٺ����ڶ�ȡ�������ṹ������B����ͬ�����š��۲���FeCl3��Һ������ɫ��Ӧ��
д����������һ��ͬ���칹��Ľṹ��ʽ ��
��5��G����Ҫ�Ĺ�ҵԭ�ϣ��û�ѧ����ʽ��ʾG��һ����Ҫ�Ĺ�ҵ��; ��
��1���Ҵ�(1��)
��2��CH3COOH��C2H5OH CH3COOC2H5��H2O (2��)
CH3COOC2H5��H2O (2��)
��3��C9H10O3 (2��) ��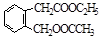 (2��) ��ˮ�ⷴӦ����ȡ����Ӧ (2��)
(2��) ��ˮ�ⷴӦ����ȡ����Ӧ (2��)
��4��3 (2��)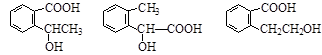 ����д����һ��
����д����һ��
�ṹ��ʽ���ɣ�2�֣�
��5�� ��CH2��CH2��H2O
��CH2��CH2��H2O CH3CH2OH��д��һ�������ķ�Ӧʽ���ɣ�2�֣�
CH3CH2OH��д��һ�������ķ�Ӧʽ���ɣ�2�֣�
��2��CH3COOH��C2H5OH
 CH3COOC2H5��H2O (2��)
CH3COOC2H5��H2O (2��)��3��C9H10O3 (2��) ��
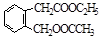 (2��) ��ˮ�ⷴӦ����ȡ����Ӧ (2��)
(2��) ��ˮ�ⷴӦ����ȡ����Ӧ (2��)��4��3 (2��)
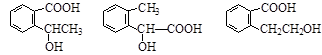 ����д����һ��
����д����һ���ṹ��ʽ���ɣ�2�֣�
��5��
 ��CH2��CH2��H2O
��CH2��CH2��H2O CH3CH2OH��д��һ�������ķ�Ӧʽ���ɣ�2�֣�
CH3CH2OH��д��һ�������ķ�Ӧʽ���ɣ�2�֣����������B��Ũ�����������������E����E�Ľṹ��֪��B����������Ӧ����E����BΪ
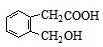 ��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��EΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ
��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��EΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ ��
����1��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH����ѧ����Ϊ�Ҵ����ʴ�Ϊ���Ҵ���
��2����Ӧ����CH3COOH��CH3CH2OH����������Ӧ����������������Ӧ����ʽΪ
CH3COOH+CH3CH2OH
 CH3COOC2H5+H2O��
CH3COOC2H5+H2O����3��������������֪��B�Ľṹ��ʽ��
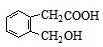 ������B�ķ���ʽ��C9H10O3��
������B�ķ���ʽ��C9H10O3��A�Ľṹ��ʽ��
 �����Է�Ӧ�ٵķ�Ӧ������ˮ�ⷴӦ��
�����Է�Ӧ�ٵķ�Ӧ������ˮ�ⷴӦ����4��
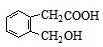 ��ͬ���칹���������3���������ٺ����ڶ�ȡ�������ṹ������B����ͬ�����ţ�����-COOH��-OH���۲���FeCl3��Һ������ɫ��Ӧ���������ǻ�������������ͬ���칹��Ϊ��
��ͬ���칹���������3���������ٺ����ڶ�ȡ�������ṹ������B����ͬ�����ţ�����-COOH��-OH���۲���FeCl3��Һ������ɫ��Ӧ���������ǻ�������������ͬ���칹��Ϊ��
��5����ϩ���Ժϳɾ���ϩ����Ӧ����ʽΪ��2CH2=CH2

 �����Ʊ��ƾ�����Ӧ����ʽΪ��CH2=CH2+H2O
�����Ʊ��ƾ�����Ӧ����ʽΪ��CH2=CH2+H2O CH3CH2OH����
CH3CH2OH����
��ϰ��ϵ�д�
�����Ŀ
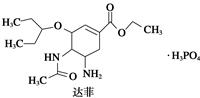
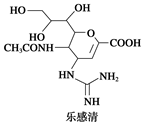
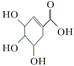
 )�������ҹ�ʢ���İ˽������С�����ç�������ʵ�����������˵����ȷ���ǣ� ��
)�������ҹ�ʢ���İ˽������С�����ç�������ʵ�����������˵����ȷ���ǣ� ��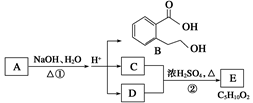
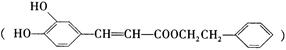 ��һ����Ȼ����ҩ���һ���������ܷ�����
��һ����Ȼ����ҩ���һ���������ܷ�����
 ��ʾ���л����У��ܷ�����ȥ��Ӧ�Ĺ���
��ʾ���л����У��ܷ�����ȥ��Ӧ�Ĺ���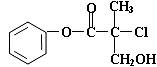


 ��
��