��Ŀ����
A��B���Ƿ����廯���1 mol Aˮ��õ�1 mol B��1 mol���ᡣA��B����Է���������������200����ȫȼ�ն�ֻ����CO2��H2O����B������C��HԪ���ܵ������ٷֺ���Ϊ65.2%(����������Ϊ0.625)��A��Һ�������ԣ�����ʹFeCl3(aq)��ɫ��
(1)Mr(A)��Mr(B)= ;
(2)1��B�����е�Oԭ�Ӹ�����______��
(3)A�ķ���ʽ��______��
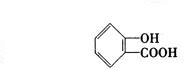
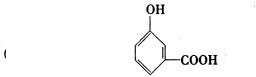
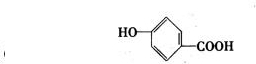
(4)B���ܵ����ֽṹ��ʽ�ֱ���______��______��______��

����:
������֪��A��H2O![]() CH3COOH��B�����������غ㶨�ɵã�Mr(A)��18=60��Mr(B)��Mr(A)��Mr(B)=60��18=42����Mr(B)=Mr(A)��42��200��42=158��
CH3COOH��B�����������غ㶨�ɵã�Mr(A)��18=60��Mr(B)��Mr(A)��Mr(B)=60��18=42����Mr(B)=Mr(A)��42��200��42=158��
�ɡ�A��Һ�������ԣ�����ʹFeCl3(aq)��ɫ��֪��A���������Ȼ�����ôB������Ҳ���Ȼ������д�A(����ij��)ˮ������ǻ���������B������������3����ԭ�ӡ���B�����к���4����ԭ�ӣ���Mr(B)=![]() =184����Mr(B)��158ì�ܣ�����ȷ��B�����к���3����ԭ�ӡ���Mr(B)=
=184����Mr(B)��158ì�ܣ�����ȷ��B�����к���3����ԭ�ӡ���Mr(B)= ![]() =138�����BΪ�����廯������֪��BΪ�ǻ�����(C7H6O3)����A����ʽΪ��C9H8O4��
=138�����BΪ�����廯������֪��BΪ�ǻ�����(C7H6O3)����A����ʽΪ��C9H8O4��

��ϰ��ϵ�д�
 �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
 ��
��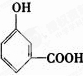 ��
��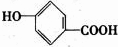 ����д���֣�
����д���֣�