��Ŀ����
��ע����ˮ����֤����������������ش������������⣺
��1������ˮ�е�NO3- �����ཡ�������Σ����Ϊ�˽�������ˮ��NO3-��Ũ�ȣ������ڼ��������������۽�NO3-��ԭΪN2���仯ѧ����ʽΪ��
10Al+6NaNO3+4NaOH=10NaAlO2+3N2��+2H2O����ش��������⣺
��������Ӧ�е�������Ϊ ��
��������Ӧ�������ɱ����3.36LN2����ת�Ƶĵ�����Ϊ ��
��2�����ֹ�������ˮ������ɱ����Ϊ���ƴ��ģ��Ⱦ�Լ�����������Ч����֮һ��Ư���dz��õ���������
�ٹ�ҵ����ȡ�����Ļ�ѧ��Ӧ����ʽΪ ��
��Ư������ˮ���ܿ����е�CO2���ã���������Ư�ס�ɱ�����õĴ����ᣬ��Ӧ�����ӷ���ʽΪ ��
�� NaNO3 �� 1.5NA��9.03 ��1023��2NaCl+2 H2O=Cl2 ��+H2 ��+2NaOH
�� Ca2++2 ClO-+CO2+H2O=CaCO3��+2 HClO
���������������1���ٷ�Ӧ����NaNO3��������N2��N�ļ�̬��+5�۱�Ϊ0�ۡ����ϼ۽��ͣ�����
��������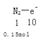 �������������Ϊ1.5NA
�������������Ϊ1.5NA
��2)��2NaCl+2 H2O=Cl2 ��+H2 ��+2NaOH
��Ca2++2 ClO-+CO2+H2O=CaCO3��+2 HclO
���㣺����������ԭ��Ӧ�еĻ���֪ʶ�͵��ӵ�ת���������㡣

����Ѫ�쵰���к���Fe2�����ӣ������ʳ�������Σ���ʹ���ж�����Ϊ�������λ�ʹFe2������ת��ΪFe3�����ӣ����ɸ���Ѫ�쵰��ɥʧ��O2��ϵ�����������ά����C�ɻ����������ε��ж�����˵��ά����C���У� ��
| A������ | B������ | C����ԭ�� | D�������� |
��Ӧ��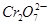 ��3
��3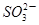 ��aH����2Cr3����3Y��4H2O����˵������ȷ����
��aH����2Cr3����3Y��4H2O����˵������ȷ����
A��Y��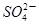 |
| B��a��8 |
C��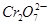 �������� �������� |
D������1 mol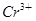 ʱת�Ƶĵ�������3��6.02��1023 ʱת�Ƶĵ�������3��6.02��1023 |
H-�Ǹ�һ�۵������ӣ�������NH3�������·�Ӧ��H��+NH3�� H2+NH2���������й������Ӧ��˵������ȷ����
| A���������û���Ӧ |
| B����Ӧ�б������ͱ���ԭ��Ԫ�ض���HԪ�� |
| C���÷�Ӧ��NH3������ |
| D���÷�Ӧ��H���������� |
С����ʵ��ʱ��С��մ��һЩKMnO4��Ƥ���ϵĺڰߺܾò�������������ò����ϡ��Һϴ�����Ͽ��Ը�ԭ�������ӷ���ʽΪ��MnO4����H2C2O4��H����CO2����Mn2������(δ��ƽ)�����ڴ˷�Ӧ��������ȷ����( )
| A���÷�Ӧ����������H2C2O4 | B��1molMnO4���ڷ�Ӧ��ʧȥ5mol���� |
| C���÷�Ӧ�ҿ��ڵIJ�����OH�� | D����ƽ�÷�Ӧ��H���ļ�������6 |
���������ǹ��ʹ��ϵĸ�Ч��ȫɱ������������ҵ�Ʊ�ClO2�ķ�Ӧԭ�������ã�
2NaClO3 �� 4HCl �� 2ClO2����Cl2����2H2O��2NaCl�����й��ڸ÷�Ӧ��˵������ȷ����
| A��Ũ�����ڷ�Ӧ�н����ֻ�ԭ�� |
| B��ÿ����0.lmol ClO2ת��0.5mol���� |
| C�������ԣ�NaClO3 <ClO2 |
| D���������ͱ���ԭ�����ʵ����ʵ���֮��Ϊ1:1 |
����������ˮ��Ӧ�����У�ˮ�õ����ӵ��ǣ� ��
| A���������� | B������ | C���� | D������ |
��֪HNO3��Ũ����S��������NO2���÷�Ӧ�ĺ������϶��������ǣ� ��
| A��H2S | B��SO2 | C��SO3 | D��H2SO4 |