��Ŀ����
��4�֣���֪2NH3(g)  N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3 ����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3 ����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
|
���� |
NH3 |
N2 |
H2 |
|
���ʵ�����mol�� |
0. 1 |
0.2 |
0.6 |
��ʱ����ƽ����Ӧ����v(N2) ��
�ﵽƽ��״̬������˵����ȷ���ǣ�
a��ͨ���ı䷴Ӧ������ʹ����ƽ���ƶ�
b��NH3�ķֽ����ʺͺϳ��������
c��NH3���ٷֽ���
d��ͨ���ı䷴Ӧ������ʹNH3ȫ���ֽ��N2�� H2
0.01moll-1min-1 ab
��������

��4�֣���֪2NH3(g) N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3 ����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
| ���� | NH3 | N2 | H2 |
| ���ʵ�����mol�� | 0. 1 | 0.2 | 0.6 |
��ʱ����ƽ����Ӧ����v(N2) ��
�ﵽƽ��״̬������˵����ȷ���ǣ�
a��ͨ���ı䷴Ӧ������ʹ����ƽ���ƶ�
b��NH3�ķֽ����ʺͺϳ��������
c��NH3���ٷֽ���
d��ͨ���ı䷴Ӧ������ʹNH3ȫ���ֽ��N2�� H2
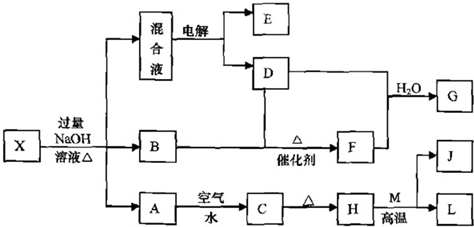
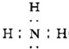
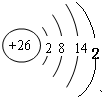
 N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£� ������ʹ����ƽ���ƶ�
������ʹ����ƽ���ƶ�