��Ŀ����
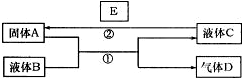
��ѧ��ѧ���кܶ����ʿ���ʵ����ͼ6-5������֮���ת�������з�Ӧ�����Ͳ��ַ�Ӧ�IJ�������ȥ��
ͼ6-5
(1)��A��һ���Ϻ�ɫ����������D��ʹƷ����Һ��ɫ������ʱ�ֿ��Իָ�ԭɫ��д����Ӧ�ٵĻ�ѧ����ʽ��______________________������D����һ������H��Ϻ�����һ�ֵ���ɫ����W��д���÷�Ӧ�Ļ�ѧ����ʽ��______________________��
(2)��A��һ�ֽ������ʣ�D����������壬B�ܷ���NaOH��Һ?___________(��ܡ���)
(3)��A�ǽ������ʣ�D��һ����ɫ���壬����������Ϊ����ɫ��Һ��C����ɫ��
д����Ӧ�ٵ����ӷ���ʽ��____________________________________________��
д����Ӧ�ڵ����ӷ���ʽ��____________________________________________��
(1)Cu+2H2SO4 (Ũ)==== CuSO4+SO2��+2H2O
2H2S+SO2====3S+2H2O
(2)��
(3)3Cu+8H++2![]() ====3Cu2++2NO+4H2O Cu2++Fe==== Fe2++Cu
====3Cu2++2NO+4H2O Cu2++Fe==== Fe2++Cu

��ϰ��ϵ�д�
 ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
�����Ŀ
 ��2007?�Ͳ���ģ����ѧ��ѧ���кܶ����ʿ���ʵ����ͼ������֮���ת�������з�Ӧ�����Ͳ��ַ�Ӧ�IJ�������ȥ��Һ��B��C�����ǵ�һ���ʵ���Һ��Ҳ�����Ǵ����
��2007?�Ͳ���ģ����ѧ��ѧ���кܶ����ʿ���ʵ����ͼ������֮���ת�������з�Ӧ�����Ͳ��ַ�Ӧ�IJ�������ȥ��Һ��B��C�����ǵ�һ���ʵ���Һ��Ҳ�����Ǵ���� 