��Ŀ����
6���������߷ֱ��ʾԪ�ص�ij��������˵�����Ĺ�ϵ��ZΪ�˵������YΪԪ�ص�ij���ʣ����������й�Ԫ�����ʵ����߱��������ӦԪ�����ʺ���������У�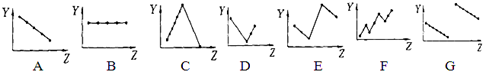
��1����A��Ԫ�ص�����������B��
��2����������Ԫ�ص�����ϼ�C��
��3��������������Na+��Mg2+��Al3+��P3-��S2-��Cl-�����Ӱ뾶E��
��4���ڶ���������Ԫ����ԭ����������ԭ�Ӱ뾶�ı仯���������������壩G��
��5��������������������������������գ�A��
���� ��1����A��Ԫ�صļ۵�����Ϊ2����˵�������۵��������䣻
��2����������Ԫ�ص�����ϼۣ���˵������������ϼ���+1�۵�����+7�������ϡ������0�۽�����
��3�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����ӵ��Ӳ�Խ�࣬���Ӱ뾶Խ��
��4��ͬ��������Ԫ�أ���ԭ����������ԭ�Ӱ뾶��С��
��5��������Խǿ�������ӵ�������Խ����
��� �⣺��1����A��Ԫ�صļ۵�����Ϊ2����˵�������۵��������䣬��ͼB���ϣ���ѡ��B��
��2����������Ԫ�ص�����ϼۣ���˵������������ϼ���+1�۵�����+7�������ϡ������0�۽�������ͼC���ϣ���ѡ��C��
��3�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС���������Ӱ뾶Na+��Mg2+��Al3+��P3-��S2-��Cl-�����ӵ��Ӳ�Խ�࣬���Ӱ뾶Խ���������Ӱ뾶Cl-��Na+���������Ӱ뾶P3-��S2-��Cl-��Na+��Mg2+��Al3+����ͼE���ϣ���ѡ��E��
��4��ͬ��������Ԫ�أ���ԭ����������ԭ�Ӱ뾶��С����ͼG���ϣ���ѡ��G��
��5��ͬ�������϶��½�������ǿ��������Խǿ�������ӵ�������Խ�����ʼ����������������������˵���������������ͼA���ϣ���ѡ��A��
���� ���⿼��ԭ�ӽṹ��Ԫ�����ʣ����ض�Ԫ�������ɵĿ��飬��������ͬһ���ڡ�ͬһ����Ԫ�����ʱ仯���ɣ������������Щ���ɽ��⣬��Ŀ�ѶȲ���

| A�� | N2���ӵĵ���ʽ��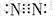 | B�� | ������Ϊ18����ԭ�ӵ�ԭ�ӷ��ţ�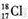 | ||
| C�� | 18O2-�Ľṹʾ��ͼ��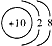 | D�� | CO2�ı���ģ�ͣ� |
 �����м���ʹKMnO4������Һ��ɫ��������ˮ��Ӧʹ��ˮ��ɫ���ǣ�������
�����м���ʹKMnO4������Һ��ɫ��������ˮ��Ӧʹ��ˮ��ɫ���ǣ�������| A�� | �ڢܢݢ� | B�� | �ڢݢ� | C�� | �ڢܢݢ� | D�� | �ڢܢݢߢ� |
| A�� | HCOOH | B�� | HCHO | C�� | CH4 | D�� | C6H12O6 |
| A�� | ������ͨ��ˮ�У�Cl2+H2O�T2H++Cl-+ClO- | |
| B�� | Ca��HCO3��2��Һ��������NaOH��Һ���2HCO3-+Ca2++2OH-�T2 H2O+CaCO3��+CO32- | |
| C�� | ������������Һ��ϡ���3Fe3O4+28H++NO3-�T9Fe3++NO��+14H2O | |
| D�� | NH4HCO3��Һ�������NaOH��Һ��Ϲ��ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O |
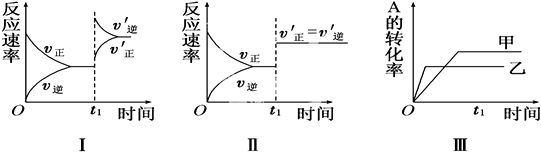
| A�� | ͼ���ʾ����t1ʱ�������¶ȶԷ�Ӧ���ʵ�Ӱ�� | |
| B�� | ͼ���ʾ��һ����t1ʱ�̼��������Է�Ӧ���ʵ�Ӱ�� | |
| C�� | ͼ���ʾ����ѹǿ�Ի�ѧƽ���Ӱ�죬���ҵ�ѹǿ�ϸ� | |
| D�� | ͼ���ʾ�����¶ȶ�ƽ���Ӱ�죬���ҵ��¶ȱȼ� |
| A�� | C8H10 | B�� | C10H16 | C�� | C12H22 | D�� | C14H22 |
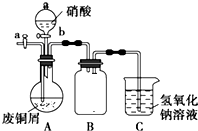 ��ʽ̼��ͭ��һ�ֻ���ԭ�ϣ���ѧʽ��mCu��OH��2•nCuCO3��ʾ��ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ�����ͼ��
��ʽ̼��ͭ��һ�ֻ���ԭ�ϣ���ѧʽ��mCu��OH��2•nCuCO3��ʾ��ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ�����ͼ�� ��
��