��Ŀ����
�л���A��C10H20O2������������ζ��������������ϴ���㲨�ķ��㸳�����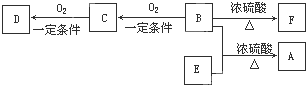
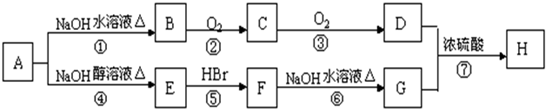
��֪��
��B������û��֧����
��D��E��Ϊ������ͬ�����ŵ�ͬ���칹�壮E���������ϵ�������Clȡ������һ�ȴ���ֻ��һ�֣�
��F����ʹ������Ȼ�̼��Һ��ɫ��
��1��B���Է����ķ�Ӧ��
��ȡ����Ӧ����ȥ��Ӧ �ۼӾ۷�Ӧ��������Ӧ
��2��д���������ʵĽṹ��ʽ��B
��3��д����D��E������ͬ�����ŵ�ͬ���칹��Ŀ��ܽṹ��ʽ����D��E�⣩��

��֪��
��B������û��֧����
��D��E��Ϊ������ͬ�����ŵ�ͬ���칹�壮E���������ϵ�������Clȡ������һ�ȴ���ֻ��һ�֣�
��F����ʹ������Ȼ�̼��Һ��ɫ��
��1��B���Է����ķ�Ӧ��
�٢ڢ�
�٢ڢ�
�� ѡ����ţ�����ȡ����Ӧ����ȥ��Ӧ �ۼӾ۷�Ӧ��������Ӧ
��2��д���������ʵĽṹ��ʽ��B
CH3��CH2��3CH2OH
CH3��CH2��3CH2OH
E��CH3��3CCOOH
��CH3��3CCOOH
��3��д����D��E������ͬ�����ŵ�ͬ���칹��Ŀ��ܽṹ��ʽ����D��E�⣩��
CH3CH��CH3��CH2COOH
CH3CH��CH3��CH2COOH
��CH3CH2CH��CH3��COOH
CH3CH2CH��CH3��COOH
��������B������������D��D�����Ȼ���ת����ϵ�ɵ�BΪ����CΪȩ��D��E��Ϊ������ͬ�����ŵ�ͬ���칹�壬D��EΪ���ᣬ��AΪ������B��C��D��E��F������̼ԭ������ͬ��A�ķ���ʽΪC10H20O2����B��E����ʽ����ΪC5H12O��C5H10O2��B��֧������B�ṹ��ʽΪCH3��CH2��3CH2OH��CΪCH3��CH2��3CHO��DΪCH3��CH2��3COOH��E���������ϵ�������Clȡ������һ�ȴ���ֻ��һ�֣���E�Ľṹ��ʽΪ��CH3��3CCOOH��B��E����������Ӧ����A����AΪC��CH3��3COOCH2��CH2��3CH3��F����ʹ������Ȼ�̼��Һ��ɫ����B��Ũ���ᡢ���������·�����ȥ��Ӧ����F��FΪϩ�����ṹ��ʽΪCH3��CH2��2CH=CH2���ݴ˽��
����⣺B������������D��D�����Ȼ�����ת����ϵ�ɵ�BΪ����CΪȩ��D��E��Ϊ������ͬ�����ŵ�ͬ���칹�壬D��EΪ���ᣬ��AΪ������B��C��D��E��F������̼ԭ������ͬ��A�ķ���ʽΪC10H20O2����B��E����ʽ����ΪC5H12O��C5H10O2��B��֧������B�ṹ��ʽΪCH3��CH2��3CH2OH��CΪCH3��CH2��3CHO��DΪCH3��CH2��3COOH��E���������ϵ�������Clȡ������һ�ȴ���ֻ��һ�֣���E�Ľṹ��ʽΪ��CH3��3CCOOH��B��E����������Ӧ����A����AΪC��CH3��3COOCH2��CH2��3CH3��F����ʹ������Ȼ�̼��Һ��ɫ����B��Ũ���ᡢ���������·�����ȥ��Ӧ����F��FΪϩ�����ṹ��ʽΪCH3��CH2��2CH=CH2��
��1��B�ṹ��ʽΪCH3��CH2��3CH2OH������-OH�����Է���ȡ����Ӧ�����ǻ�������̼ԭ�����ڵ�̼ԭ���Ϻ���Hԭ�ӣ����Է�����ȥ��Ӧ�����ǻ�������̼ԭ���Ϻ���Hԭ�ӣ����Է������������ܷ����Ӿ۷�Ӧ��
�ʴ�Ϊ���٢ڢܣ�
��2��������������֪��BΪCH3��CH2��3CH2OH��EΪ��CH3��3CCOOH��
�ʴ�Ϊ��CH3��CH2��3CH2OH����CH3��3CCOOH��
CH3��CH2��3COOH�����еĹ�����Ϊ�Ȼ���FΪCH3��CH2��2CH=CH2�����������Ĺ�������̼̼˫����
�ʴ�Ϊ���Ȼ���̼̼˫����
��3����CH3��CH2��3COOH����CH3��3CCOOH������ͬ�����ŵ�ͬ���칹�壬�������ᣬ���ܽṹ��ʽ�У�
CH3CH��CH3��CH2COOH��CH3CH2CH��CH3��COOH��
�ʴ�Ϊ��CH3CH��CH3��CH2COOH��CH3CH2CH��CH3��COOH��
��1��B�ṹ��ʽΪCH3��CH2��3CH2OH������-OH�����Է���ȡ����Ӧ�����ǻ�������̼ԭ�����ڵ�̼ԭ���Ϻ���Hԭ�ӣ����Է�����ȥ��Ӧ�����ǻ�������̼ԭ���Ϻ���Hԭ�ӣ����Է������������ܷ����Ӿ۷�Ӧ��
�ʴ�Ϊ���٢ڢܣ�
��2��������������֪��BΪCH3��CH2��3CH2OH��EΪ��CH3��3CCOOH��
�ʴ�Ϊ��CH3��CH2��3CH2OH����CH3��3CCOOH��
CH3��CH2��3COOH�����еĹ�����Ϊ�Ȼ���FΪCH3��CH2��2CH=CH2�����������Ĺ�������̼̼˫����
�ʴ�Ϊ���Ȼ���̼̼˫����
��3����CH3��CH2��3COOH����CH3��3CCOOH������ͬ�����ŵ�ͬ���칹�壬�������ᣬ���ܽṹ��ʽ�У�
CH3CH��CH3��CH2COOH��CH3CH2CH��CH3��COOH��
�ʴ�Ϊ��CH3CH��CH3��CH2COOH��CH3CH2CH��CH3��COOH��
�����������л�����ƶϣ��漰����ȩ�������������ת���ȣ��Ѷ��еȣ�������Ŀ��Ϣ����Ϸ�Ӧ���������ƶϣ�ȷ��B��C��D��E��F������̼ԭ������ͬ�ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
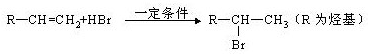
 ���ܷ�����������Ӧ��
���ܷ�����������Ӧ��