��Ŀ����
A��B��C��D��E���ֶ�����Ԫ�ص�ԭ������������������Ԫ����ֻ��һ�ֽ���Ԫ�أ�A��Bͬ���ڣ�������AB2��DB2���ǿ�ʹ����ʯ��ˮ����ǵ����壮����֪C��E�ĵ��ʾ�������NaOH��Һ����C��NaOH��Һ��Ӧ�ɲ������壮��1��д��D��E����Ԫ�ص����ƣ�A��______ B��______ C��______D��______��E��______��
��2��д��AB2 �ĵ���ʽ��______
��3��д��DB2ʹ����ʯ��ˮ����ǵĻ�ѧ����ʽ��______��
��4��д��C��E�ĵ��ʷֱ���NaOH��Һ��Ӧ�����ӷ���ʽ��______��______��
���𰸡�������A��Bͬ���ڣ�������AB2��DB2���ǿ�ʹ����ʯ��ˮ����ǵ����壬����������CO2��SO2�����֪AΪCԪ�أ�BΪOԪ�أ�DΪSԪ�أ�C��E�ĵ��ʾ�������NaOH��Һ����C��NaOH��Һ��Ӧ�ɲ������壬ԭ����������8��O����16��S��֮���ֻ��Al���ϣ���CΪAl������EΪCl��Ϊԭ�������������������Ԫ�أ�����Ԫ�ض�Ӧ�ĵ��ʡ�����������ʽ����⣮
����⣺��1��A��Bͬ���ڣ�������AB2��DB2���ǿ�ʹ����ʯ��ˮ����ǵ����壬����������CO2��SO2�����֪AΪCԪ�أ�BΪOԪ�أ�DΪSԪ�أ�C��E�ĵ��ʾ�������NaOH��Һ����C��NaOH��Һ��Ӧ�ɲ������壬ԭ����������8��O����16��S��֮���ֻ��Al���ϣ���CΪAl������EΪCl��
�ʴ�Ϊ��̼�������������ȣ�
��2��AB2ΪCO2��Ϊ���ۻ�����������и�ԭ�ӵ������ﵽ8�����ȶ��ṹ������ʽΪ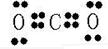 ��
��
�ʴ�Ϊ��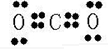 ��
��
��3��SO2�����ʯ��ˮ��Ӧ��������ˮ��CaSO3����Ӧ�Ļ�ѧ����ʽΪSO2+Ca��OH��2�TCaSO3��+H2O��
�ʴ�Ϊ��SO2+Ca��OH��2�TCaSO3��+H2O��
��4��Al��NaOH��Һ��Ӧ����NaAlO2����������Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O�T2AlO2-+3H2����Cl2��NaOH��Һ��������������ԭ��Ӧ����NaCl��NaClO����Ӧ�����ӷ���ʽΪ
Cl2+2OH-�TClO-+Cl-+H2O���ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2����Cl2+2OH-�TClO-+Cl-+H2O��
���������⿼��Ԫ�ص��ƶϣ���Ŀ�ѶȲ���������ʵ����ʵĽǶ����ֿ���Ԫ�ص����࣬ע�ⳣ��Ԫ�ػ���������ʣ�ע�ص���ʽ�Լ����ӷ���ʽ����д��
����⣺��1��A��Bͬ���ڣ�������AB2��DB2���ǿ�ʹ����ʯ��ˮ����ǵ����壬����������CO2��SO2�����֪AΪCԪ�أ�BΪOԪ�أ�DΪSԪ�أ�C��E�ĵ��ʾ�������NaOH��Һ����C��NaOH��Һ��Ӧ�ɲ������壬ԭ����������8��O����16��S��֮���ֻ��Al���ϣ���CΪAl������EΪCl��
�ʴ�Ϊ��̼�������������ȣ�
��2��AB2ΪCO2��Ϊ���ۻ�����������и�ԭ�ӵ������ﵽ8�����ȶ��ṹ������ʽΪ
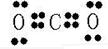 ��
���ʴ�Ϊ��
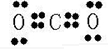 ��
����3��SO2�����ʯ��ˮ��Ӧ��������ˮ��CaSO3����Ӧ�Ļ�ѧ����ʽΪSO2+Ca��OH��2�TCaSO3��+H2O��
�ʴ�Ϊ��SO2+Ca��OH��2�TCaSO3��+H2O��
��4��Al��NaOH��Һ��Ӧ����NaAlO2����������Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O�T2AlO2-+3H2����Cl2��NaOH��Һ��������������ԭ��Ӧ����NaCl��NaClO����Ӧ�����ӷ���ʽΪ
Cl2+2OH-�TClO-+Cl-+H2O���ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2����Cl2+2OH-�TClO-+Cl-+H2O��
���������⿼��Ԫ�ص��ƶϣ���Ŀ�ѶȲ���������ʵ����ʵĽǶ����ֿ���Ԫ�ص����࣬ע�ⳣ��Ԫ�ػ���������ʣ�ע�ص���ʽ�Լ����ӷ���ʽ����д��

��ϰ��ϵ�д�
�����Ŀ






 Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������
Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������