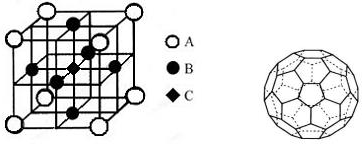
��Ŀ����
2��ʵ������Ҫ 0.1mol/L�� H2SO4��Һ980mL������ 98%���ܶ�Ϊ1.84g/cm3��Ũ�������ƣ���1���������Ҫ��������Ͳ���ձ����������⣬����Ҫ1000mL����ƿ����ͷ�ιܣ�
��2����ʵ���������ɷ�Ϊ���¼�����
A������Ͳ��ȡ5.4mLŨ���ᣬ����ע��װ��Լ50mL����ˮ���ձ�����ò��������裮
B������������ˮ������ϴ���ձ��Ͳ���������ÿ�ε�ϴҺ����������ƿ�
C����ϡ�ͺ������С�ĵ��ò�������������ƿ�
D���������ƿ�Ƿ�©ˮ��
E��������ˮֱ�Ӽ�������ƿ����Һ��ӽ��̶���1-2cm����
F���ǽ�ƿ���������ߵ���ҡ����Һ��
G���ý�ͷ�ι�������ƿ����μ�������ˮ����Һ����͵�ǡ����������У�
��ݴ���д��
��������������еĿհ״���
�ڲ��������ȷ�IJ���˳������ĸ��д����
�� D ������ A ������ C ����B��E��G���� F ����
�۽�Ũ�������ձ���ϡ�ͺ���������ƿʱ��������ȴ�����·�����Һ��
��3���Է������в�����������Һ��Ũ���к�Ӱ��
����ȡŨ����ʱ�۾�������Ͳ�̶��ߣ��ᵼ��������ҺŨ�Ȼ�ƫ�ͣ����ƫ�ߡ�ƫ�͡�����Ӱ�죬��ͬ��
�ڶ���ʱ���۾����ӿ̶��ߣ�������ҺŨ�Ȼ�ƫ�ͣ�
���� ��1������ʵ������980mL����ƿ����Ӧѡ��1000mL����ƿ��Ȼ�����������Һ�IJ���Ϊ���㡢��ȡ��ϡ�͡���Һ����ȴ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�����������������
��2���ٸ���C=$\frac{1000�Ѧ�}{M}$���Ũ��������ʵ���Ũ�ȣ�Ȼ�����ϡ�Ͷ�����������Ũ����������
�ڸ���������Һ�IJ���Ϊ���㡢��ȡ��ϡ�͡���Һ����ȴ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ȷ������˳��
��Ũ����ϡ�ͷ��ȣ�������ƿ�������ȣ�
��3������C=$\frac{n}{V}$ͨ���������ʵ����ʵ�������Һ�������������Ũ�ȵ�Ӱ�죮
��� ��1������ʵ������980mL����ƿ����Ӧѡ��1000mL����ƿ��Ȼ�����������Һ�IJ���Ϊ���㡢��ȡ��ϡ�͡���Һ����ȴ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪���������Ϊ��Ͳ���ձ�����������1000mL����ƿ����ͷ�ιܣ�����Ҫ��������Ͳ���ձ����������⣬����Ҫ������Ϊ1000mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��1000mL����ƿ����ͷ�ιܣ�
��2����Ũ��������ʵ���Ũ��C=$\frac{1000�Ѧ�}{M}$=$\frac{1000��1.84��98%}{98}$=18.4mol/L������ʵ������980mL����ƿ����Ӧѡ��1000mL����ƿ�������Ƴ�1000mL��Һ���������Ũ��������ΪVmL������ϡ�Ͷ��ɿ�֪��18.4mol/L��VmL=0.1mol/L��1000mL�������V=5.4mL���ʴ�Ϊ��5.4��
Ũ����������
�ڸ���������Һ�IJ���Ϊ���㡢��ȡ��ϡ�͡���Һ����ȴ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪������˳��Ϊ��DACBEGF���ʴ�Ϊ���� B ������ E ������ G ����
��Ũ����ϡ�ͷ��ȣ�������ƿ�������ȣ��ʱ��뽫ϡ�ͺ����Һ��ȴ�����·�����Һ���ʴ�Ϊ����ȴ�����£�
��3������ȡŨ����ʱ�۾�������Ͳ�̶��ߣ��ᵼ������ȡ��Ũ��������ƫС����������ҺŨ��ƫС���ʴ�Ϊ��ƫ�ͣ�
�ڶ���ʱ���۾����ӿ̶��ߣ�������Һ�����ƫ������ҺŨ�Ȼ�ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
���� ���⿼��������һ�����ʵ���Ũ����Һ�IJ��衢������ѡ����������������⣬Ӧע���������ƿ����ѡ���ѶȲ���

| A�� | �ڼס������ձ�����Һ�У�������Cu2+��K-��H+��Cl-��CO32-��OH-�������ӣ���֪���ձ�����Һ������3�����Ӳ�������ɫ�������ձ�����Һ�к��е�3�������ǣ�K+��OH-��CO32- | |
| B�� | ������pH=7����Һ�У�Fe3+��Mg2+��SO42-��Cl-�ܴ������� | |
| C�� | �ں���HCO3-��SO32-��S2-��CH3COO-���������ӵ���Һ�м���������BaO2�����CH3COO-Ũ�ȱ仯��С | |
| D�� | ������ˮ�������c��OH-��=10-10����Һ�У�Na+��ClO-��S2-��NH4+���ܴ������� |
��Ũ�����ϡ��
�ڳ�ȡһ��������NaOH����
������100ml0.1mol/l��NaCl��Һ
����CCl4��ȡ��ˮ��I2
��������ˮ��ȡ����ˮ��
һ��Ҫ�õ����������ǣ�������
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �ۢ� | D�� | �٢� |
| A�� | ���������ӵĵ���ʽ�� 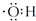 | |
| B�� | ������̼���ӵı���ģ����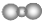 | |
| C�� | �����������ͨʽ��CnH2n-6��n��6�� | |
| D�� | 12C��14C��ԭ�ӽṹʾ��ͼ���ɱ�ʾΪ 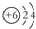 |
| A�� | ������Ь | B�� | ���г���̥ | ||
| C�� | ������������ϻ��� | D�� | ���ٶ������ϲ��� |
| A�� | Ԫ�طǽ����ԣ����� | B�� | ���������ԣ����� | ||
| C�� | �⻯���ȶ��ԣ�����ǿ | D�� | ��ۺ��������ԣ����� |
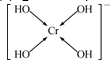 ����������λ�����ü��ű�ʾ��
����������λ�����ü��ű�ʾ��