��Ŀ����
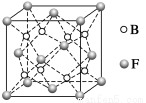
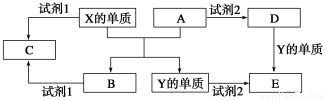
A��YΪ�����������ʣ�XΪ�����ǽ������ʡ�������X��G��H��IΪ���壬CΪҺ�塣B��������Ԫ����ɵ��Σ�����ʱ�����ֽ������������壬��ȴ���ֿɻ��ϵõ�B���й�����֮���ת����ϵ����ͼ(���ַ�Ӧ��������������ȥ)��
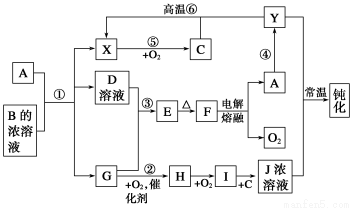
����д���пհף�
(1)B�ĵ���ʽΪ____________________________��
(2)����A��ʯī���缫��B��Ũ��Һ���������Һ������ԭ��ء���������ӦʽΪ____________________��
(3)��Ӧ���Ļ�ѧ����ʽΪ__________________________����Ӧ����ұ��ҵ������________________(�������ұ������)��
(4)��D�Ľᾧˮ�����Ʊ�D����ˮ����IJ���Ϊ_____________________________��
(5)��Ӧ���Ļ�ѧ����ʽΪ________________________________________________��
��Ӧ�������ӷ���ʽΪ___________________________________________________��
(1) 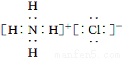
(2)2NH4+��2e��=2NH3����H2��
(3)3Fe��4H2O(g)  Fe3O4��4H2���Ȼ�ԭ�������Ȼ�ԭ��
Fe3O4��4H2���Ȼ�ԭ�������Ȼ�ԭ��
(4)��D�Ľᾧˮ������HCl�����м���
(5)4NH3��5O2 4NO��6H2O��Al3����3NH3��H2O=Al(OH)3����3NH4+ [��Al3����3NH3��3H2O=Al(OH)3����3NH4+]
4NO��6H2O��Al3����3NH3��H2O=Al(OH)3����3NH4+ [��Al3����3NH3��3H2O=Al(OH)3����3NH4+]
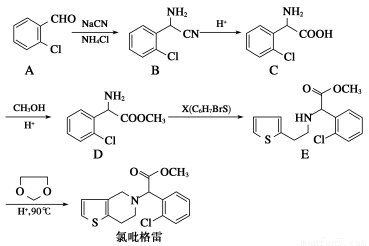
������������������ṩ��Ϣ��CΪH2O��BΪNH4Cl������AΪ���ý�����Ϊ��ⷨ�Ʊ�ʱ��O2���ɣ�AӦΪAl��Al��NH4Cl��Һ��Ӧ����X(H2)��D(AlCl3)��G(NH3)������HΪNO��IΪNO2��JΪHNO3��
AlCl3��NH3��Ӧ����Al(OH)3(E)��FΪAl2O3����������ŨHNO3�жۻ��ij�Al�⣬����Fe��ͨ�����ȷ�Ӧ������Fe(Y)������H2O(g)��Ӧ����H2��
(2)Al��ʯī���缫��NH4ClΪ�������Һ������ԭ��أ������������ӵõ��ӣ��缫��ӦʽΪ2NH4+��2e��=2NH3����H2����
(4)��AlCl3�������Ʊ���ˮAlCl3��Ϊ�˷�ֹˮ�⣬Ӧ��HCl�����м��ȡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���֪������Ԫ�ؼס��ҡ������������ԭ�����������������⻯���мס��ҡ�����������Ļ��ϼ����£�����˵����ȷ���ǣ� ��
Ԫ�� | �� | �� | �� | �� | �� |
���ϼ� | ��4 | ��1 | ��4 | ��2 | ��1 |
A���ҵij���������ֻ��һ��
B����̬�⻯���ȶ��ԣ�������
C��������������������⻯���ˮ��Һ��Ӧ
D��ԭ�Ӱ뾶��С���주��