��Ŀ����
����Ŀ�����������ѳ�Ϊ���ͬ��ע�Ļ��⣬ȼú����������β���г����е�NOx��SO2��H2S����Ⱦ������γ����꣬�ƻ����������ۺ������ǵ�ǰ��Ҫ���о����⡣
���������Ƕ���������������ۣ����в���ȷ����__________
a��ɱ��ˮ�еĸ��������������ʳ�����Դ���ƻ�ˮ����̬ϵͳ
b���Ե��ߡ����졢���������ݵȾ������������
c�����³�����ն�
d��������̼�Ĺ����ŷţ����γ��������Ҫԭ��֮һ
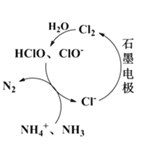
������ⷨ����������������нϸߵĻ���Ч��;���Ч�棨ͼ�е缫��Ϊʯī����

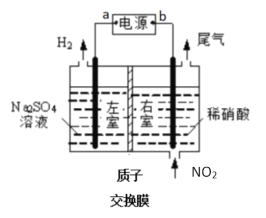
��1�����NO�Ʊ�NH4NO3ԭ�����Ϸ���ͼ��ʾ��
������Ϊʯī_____(��1��2)���õ缫�ķ�ӦʽΪ_____________��
��Ϊʹ���ĸ�����Ҳ��ȫת��ΪNH4NO3����Ҫ���������X�Ļ�ѧʽΪ___________��
��2�����Ϸ���ͼװ�ý���ģ����NO2����ʵ�飬�ɻ������ᡣ
����ӵ�Դa��Ϊ______�������ҷ����ĵ缫��ӦʽΪ_____________��
�����б�״����2.24L NO2�����գ�ͨ�����ӽ���Ĥ(ֻ��������ͨ��)��H+Ϊ______mol��
���ú�NO��NO2(������NO2ת��ΪN2O4)�ķ�������ģ���ⷨ����ʵ�顣�������У��в���NOת��ΪHNO2��ʵ�����ʱ�����������Һ��������1 mol HNO3��0.1 mol HNO2��ͬʱ�����ռ�����״����28L H2��ԭ������NO��NO2�������Ϊ____________��
���𰸡�cd 2 NO-3e-+2H2O=NO3-+4H+ NH3 �� 2H++2e- = H2������2H2O+2e- = H2��+2OH-�� 0.1 8��3
��������
I.������������ʽ����жϣ�
��.(1)���NO�Ʊ�NH4NO3ʱ������������NOʧ���ӵ�������Ӧ������������NO�õ��ӵĻ�ԭ��Ӧ�����ݵ缫��Ӧ�ж���Ҫ���������X��
(2)�ٸ���ͼ֪�����ʱ�������е缫�������ӷŵ�����������������Ϊ�����ң�����Ϊ�����ң�������ͨ����ǵ���������ɵ������ᣬ���������ϵ�������ʧ���Ӻ�ˮ���������n(NO2)=0.1mol��������ӦʽΪNO2-e-+H2O=NO3-+2H+����0.2mol���������ɣ���Ϊ��0.1mol�������ɣ��ݴ˷����жϣ��۸����������������ת�Ƶĵ�����������������1mol HNO3��0.1molHNO2���ɣ���������������������ת�Ƶĵ��ӵ����ʵ������ٽ��Nԭ���غ㼰ת�Ƶ����غ���ʽ���㡣
��a�������к����ᡢ���ᣬ������н�ǿ�����ԣ����е��������ǿ�����ԣ���ɱ��ˮ�еĸ��������������ʳ�����Դ���ƻ�ˮ����̬ϵͳ����a��ȷ��b��������н�ǿ�����ԣ����е��������ǿ�����ԣ����ߡ������к��н���ͭ�����ȣ������������к��н��������Σ����Ƕ��ܺ������еijɷַ�Ӧ����b��ȷ��c�����³�����ն����Ƿ��������������꣬��c����d.������������������Ĺ����ŷ����γ��������Ҫԭ������̼�����γ������ԭ��d����ѡcd��
��(1)�ٸ���ͼʾ�����ʱ�������е缫��NO�ŵ�����NH4+���缫��ӦʽΪNO+5e-+6H+=NH4++H2O��������Ϊ�����ң�����Ϊ�����ң�������ͨ���NO������������������ӣ�����ʯī2Ϊ�����������ϵ缫��ӦʽΪ��NO-3e-+2H2O=NO3-+4H+���ʴ�Ϊ��2��NO-3e-+2H2O=NO3-+4H+��
��1molNO�ŵ�����1molNH4+��ת��5mol���ӣ�1molNO������������������ӣ�ת��3mol���ӣ����ݵ�ʧ�����غ㣬���ɵ���������ӵ����ʵ�������笠����ӣ���ϵ缫��Ӧʽ��Ϊʹ��������ȫת��ΪNH4NO3����Ҫ���䰱�����ʴ�Ϊ��NH3��
(2)�ٸ���ͼ֪�����ʱ�������е缫�������ӷŵ�����������������Ϊ�����ң���ӵ�Դa��Ϊ���������ҷ����ĵ缫��ӦʽΪ2H++2e- = H2��������Ϊ�����ң�������ͨ����ǵ���������ɵ������ᣬ���������ϵ�������ʧ���Ӻ�ˮ�������ᣬ�缫��ӦʽΪNO2-e-+H2O=NO3-+2H+���ʴ�Ϊ������2H++2e- = H2����
��n(NO2)=![]() =0.1mol��������ӦʽΪNO2-e-+H2O=NO3-+2H+����0.2mol���������ɣ���Ϊ��0.1mol�������ɣ�����0.1mol������ͨ�����ӽ���Ĥ���������ң��ʴ�Ϊ��0.1��
=0.1mol��������ӦʽΪNO2-e-+H2O=NO3-+2H+����0.2mol���������ɣ���Ϊ��0.1mol�������ɣ�����0.1mol������ͨ�����ӽ���Ĥ���������ң��ʴ�Ϊ��0.1��
�۱�״����28L H2�����ʵ���n(H2)=![]() =1.25mol��ת�Ƶ��ӵ����ʵ���=1.25mol��2=2.5mol�����������Һ��������1 mol HNO3��0.1 mol HNO2��˵������������1mol HNO3��0.1molHNO2���ɣ�����Nԭ���غ��n(NO)+n(NO2)=1mol+0.1 mol=1.1 mol������0.1mol������ת�Ƶ���0.1mol������������ת�Ƶ������ʵ���=2.5mol-0.1mol=2.4mol���跴Ӧ���������NO���ʵ���Ϊxmol���������������ʵ���Ϊymol������Nԭ���غ㼰ת�Ƶ����غ�ã�x+y=1.1-0.1��3x+y=2.4�����x=0.7��y=0.3��n(NO)��n(NO2)=(0.7+0.1)mol��0.3mol=8��3����ͬ��������������֮�ȵ������ʵ���֮�ȣ�����NO�Ͷ������������֮��Ϊ8��3���ʴ�Ϊ��8��3��
=1.25mol��ת�Ƶ��ӵ����ʵ���=1.25mol��2=2.5mol�����������Һ��������1 mol HNO3��0.1 mol HNO2��˵������������1mol HNO3��0.1molHNO2���ɣ�����Nԭ���غ��n(NO)+n(NO2)=1mol+0.1 mol=1.1 mol������0.1mol������ת�Ƶ���0.1mol������������ת�Ƶ������ʵ���=2.5mol-0.1mol=2.4mol���跴Ӧ���������NO���ʵ���Ϊxmol���������������ʵ���Ϊymol������Nԭ���غ㼰ת�Ƶ����غ�ã�x+y=1.1-0.1��3x+y=2.4�����x=0.7��y=0.3��n(NO)��n(NO2)=(0.7+0.1)mol��0.3mol=8��3����ͬ��������������֮�ȵ������ʵ���֮�ȣ�����NO�Ͷ������������֮��Ϊ8��3���ʴ�Ϊ��8��3��

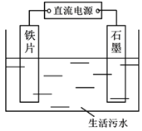
����Ŀ��I����1����H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ��д����Ӧ�����ӷ���ʽ��_______________________��
��2����������������ͬ��ӡˢ��·��Ľ�����ĩ��10%H2O2��3.0 mol/L��H2SO4�����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ�����(���±�)��
�� ��(T) | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
ͭƽ���ܽ�������10-3mol��L-1��min-1 | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ����________________________________________________��
��3�����ᴿ���CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ�����ȣ�����CuCl�������Ʊ�CuCl�����ӷ���ʽ��_________________________________________��
II��������ˮ�е��͵���Ҫ�������κ������ʽ���ڡ�
��1������ԭ������ͼ��ʾ�����õ�ⷨ��PO43-ת��ΪFe3��PO4��2������ȥ������___________������������������������û�ѧ�����ʾ����Fe2+����Ҫ���̣�__________________________��
��2����Cl-����ʱ������ԭ����ͼ��ʾ����Ҫ��������������Ч�ȣ�HClO��ClO-����NH4+��NH3����ΪN2����pH��7ʱ����Ҫ����HClO����NH4+�ķ�Ӧ�������ӷ���ʽΪ��_________________________________��