��Ŀ����
�Ӻ�ˮ����Br-Ϊ67 g��m-3������ȡBr2�Ĺ��̿ɲ�����ͼ��ʾ����ˮ![]() �ữ
�ữ![]() ����
����![]() �������塢�����������
�������塢�����������![]() ����
����![]() ����
����![]() ��
��
����������⣺
��1��д��Cl2��������������Br-�ķ�Ӧ�����ӷ���ʽ________________________ ��
��2��ΪʲôҪ�����������¶������ڼ�����������Br-��
��3�����������ɵ��嵥��Ϊʲô�������ȿ���������
��4���ȿ���������Br2��SO2��ˮ���գ�д����һ��Ӧ�Ļ�ѧ����ʽ__________________��
��1��Cl2+2Br-====2Cl-+Br2 ��2����ΪCl2����OH-����Cl-��ClO-�� ��3��Br2�ӷ��� ��4��Br2+SO2+2H2O====2HBr+H2SO4
������Cl2��������ǿ��Br2��Cl2���廯�ﷴӦ���û����塣

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 =2.6��10-39�� Mg(OH)2���ܶȻ�����
=2.6��10-39�� Mg(OH)2���ܶȻ����� ��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��
��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��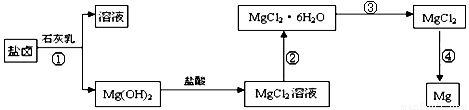
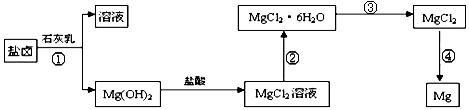
 =2.6��10-39��Mg(OH)2���ܶȻ�����
=2.6��10-39��Mg(OH)2���ܶȻ����� ��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��
��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��
 .���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������
.���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������