��Ŀ����
��16�֣�
��1����֪298Kʱ��Fe(OH)3���ܶȻ����� =2.6��10-39��Mg(OH)2���ܶȻ�����
=2.6��10-39��Mg(OH)2���ܶȻ����� =5.6��
=5.6�� ��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��
��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��
��û��Mg(OH)2��������______________��С����ޡ�����������________________________________��
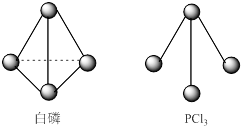
��2����HnA���B(OH)m��ȫ��Ӧ��������.
����HnAΪHCl���Ҹ�����Һ��pH��7�������ӷ���ʽ˵��ԭ��
������0.4mol��L��1��NaOH��Һ��0.2mol��L��1��HnA��Һ�������Ϻ�pH=10��
��HnAΪ ������ţ�.
a.һԪǿ�� b. һԪ���� c. ��Ԫǿ�� d. ��Ԫ����
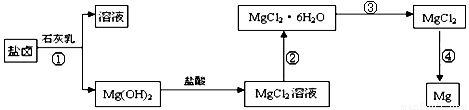
��3��ijѧУ������ȤС��Ӻ�ˮɹ�κ����±(��Ҫ��Na+��Mg2+��Cl����Br����)��ģ�ҵ��������ȡþ����Ҫ�������£��ش��������⣺
��.�ӹ��̢ٵõ���Mg(OH)2�����л���������Ca(OH)2 ����ȥ����Ca(OH)2�ķ������Ƚ��������뵽ʢ�� ��Һ���ձ��У���ֽ�����ˡ�ϴ�ӿɵô�����Mg(OH)2��
�� .���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������
.���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������
��.д�����̢��з�����Ӧ�Ļ�ѧ����ʽ
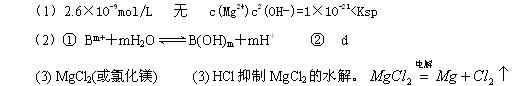
����

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д�I.����ͼ��ʾ����2molA�����1molB�������һ�ݻ��ɱ���ܱ������С�
������Ӧ��2A(g)+B(g)![]() 2C(g)����Ӧ��ʼʱ�ɻ����Ļ�����λ����ͼ1��ʾ������Ӧ�ﵽƽ��ʱ������λ����ͼ2��ʾ.��ﵽƽ��ʱ��A��ת����Ϊ________________;
2C(g)����Ӧ��ʼʱ�ɻ����Ļ�����λ����ͼ1��ʾ������Ӧ�ﵽƽ��ʱ������λ����ͼ2��ʾ.��ﵽƽ��ʱ��A��ת����Ϊ________________;
�������·�Ӧ��ƽ�ⳣ��Ϊ_______________________________��
|
|

|
|
|
|
|
��.��1����֪298Kʱ��1molC2H6����������ȫȼ�����ɶ�����̼��Һ̬ˮ���ų�����1558.3KJ��д���÷�Ӧ�Ļ�ѧ����ʽ__________________________________________.
 ��20���ø÷�Ӧ���һ��ȼ�ϵ��:������������Һ����� ����Һ���ö��ʯī���缫���ڵ缫�Ϸֱ���������������д�������ĵ缫��Ӧʽ____________________________
��20���ø÷�Ӧ���һ��ȼ�ϵ��:������������Һ����� ����Һ���ö��ʯī���缫���ڵ缫�Ϸֱ���������������д�������ĵ缫��Ӧʽ____________________________
![]() (3)�����һ�ѧʵ��װ��ͼ��
(3)�����һ�ѧʵ��װ��ͼ��
ʯī���ϵĵ缫��ӦʽΪ_______________________________;
�����ʼʱʢ��1000mLpH=5������ͭ��Һ��25�棩��CuSO4 ��������һ��ʱ�����Һ��pH��Ϊ1����Ҫʹ��Һ�ָ�����ʼŨ�ȣ�������Һ����ı仯����������Һ�м���________(����������)��������Ϊ_______________.


 =2.6��10-39�� Mg(OH)2���ܶȻ�����
=2.6��10-39�� Mg(OH)2���ܶȻ����� ��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��
��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��