��Ŀ����
������� ��K2FeO4����һ�����͡���Ч�����ˮ���������DZ�Cl2��O3��ClO2��KMnO4�����Ը�ǿ��������Ⱦ����ɫˮ����������ҵ�����Ƶø������ƣ�Ȼ���ڵ����£��ڸ���������Һ�м���KOH�����;Ϳ�����������أ�K2FeO4����
ʪ���Ʊ�����Ҫ��Ӧ����Ϊ��2Fe(OH)3 +3ClO��+4OH�� =2FeO42�� +3Cl�� +5H2O ,
�ɷ��Ʊ�����Ҫ��Ӧ����Ϊ��2FeSO4 +6Na2O2 = 2Na2FeO4 +2Na2O +2Na2SO4 +O2��
�����й�˵������ȷ���ǣ��� ��
ʪ���Ʊ�����Ҫ��Ӧ����Ϊ��2Fe(OH)3 +3ClO��+4OH�� =2FeO42�� +3Cl�� +5H2O ,
�ɷ��Ʊ�����Ҫ��Ӧ����Ϊ��2FeSO4 +6Na2O2 = 2Na2FeO4 +2Na2O +2Na2SO4 +O2��
�����й�˵������ȷ���ǣ��� ��
| A���������������+6�� |
| B��ʪ����ÿ����1mol Na2FeO4ת��3mol���� |
| C���ɷ���ÿ����1 mol Na2FeO4ת��4mol���� |
| D��K2FeO4����ˮʱ������������ɱ�������ܳ�ȥˮ���е�H2S��NH3�ȣ����ɵ�Fe(OH)3���廹������ˮ�е��������� |
C
��

��ϰ��ϵ�д�
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
�����Ŀ
 _��____����������Һ�м��뼸���Ȼ�����Һ���۲쵽��������____��____��
_��____����������Һ�м��뼸���Ȼ�����Һ���۲쵽��������____��____�� �չ�������___��____���壨�����ƻ���ţ����ɣ���Ӧ�и��������____��____(���������������ԭ����)
�չ�������___��____���壨�����ƻ���ţ����ɣ���Ӧ�и��������____��____(���������������ԭ����) H4��Ϊȼ�ϣ�����ƽ�䷴Ӧ�ķ���ʽ��
H4��Ϊȼ�ϣ�����ƽ�䷴Ӧ�ķ���ʽ��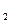 ��������̼ͨ��ʯ��ˮ��
��������̼ͨ��ʯ��ˮ��
 ��
��
