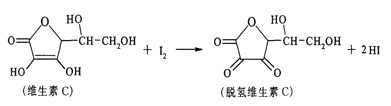
��Ŀ����
����Ŀ������Ӧ����㷺�Ľ��������������±�����Լ������ξ�Ϊ��Ҫ�����
��1��������Ϊ����ɫ��ĩ��������������ˮ�������������У���д�������������ᷴӦ�����ӷ���ʽ______________________________��
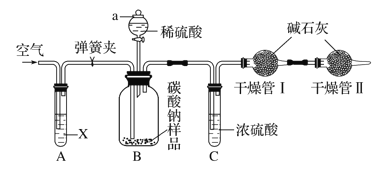
��2�����������о���ȼ�գ���������ɫ���̣���ȼ�����õ����Ȼ�������ˮ��������Һ����������Ϊ16.25%�����ܶ�Ϊ6.0g�� mL-1�������Һ�������ӵ����ʵ���Ũ��Ϊ________________�������������Ƶ���Һϡ��Ϊ0.15mol/L ��ϡ��Һ480mL����Ҫ���������ձ�������������Ͳ����ͷ�ι�֮�⣬����Ҫ________________����ȡ����Һ�������_______________mL�������ƹ������������ʱ���ӿ̶��ߣ������������ҺŨ��________������ƫ��������ƫ����������Ӱ��������
��3��������FeCl3��Һ������ڵ�����ˮ�У������Ƶ�Fe��OH��3���壬��д���ù��̵Ļ�ѧ��Ӧ����ʽ_______________________�����¹ض�Fe��OH��3�����˵���в���ȷ����_____________���������
A. Fe��OH��3������һ�ֺ��ɫ�����塢���Ļ����
B. Fe��OH��3�����з�ɢ�ʵ���ֱ����10-9m~10-7m ֮��
C.������������Fe��OH��3�����FeCl3 ��Һ���ö����ЧӦ����Fe��OH��3�����FeCl3 ��Һ
D.ȡ����Fe��OH��3���������Թ��У����Թ�����εμ�ϡ���ᣬ�ɿ����Ȳ������ɫ�������������ܽ⣬���յõ���ɫ����Һ
E.����װ��U �ι��ڣ���ʯī���缫����ֱͨ���磬ͨ��һ��ʱ����ֿ�����������������ɫ����
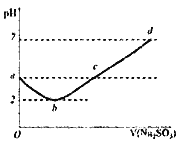
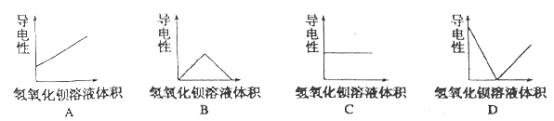
��4������������Һ�У���μ����Ũ�ȵ�����������Һ������Һ�ĵ�����������������Һ������Ӷ��仯��ͼ������_______��
���𰸡� Fe2O3+6H��==2 Fe3��+3H2O 18 mol��L��1 500 ml����ƿ 12.5 ƫ�� FeCl3+3 H2O![]() Fe��OH��3�������� +3HCl CE D
Fe��OH��3�������� +3HCl CE D
����������1�������������ᷴӦ�����Ȼ�����ˮ����Ӧ�����ӷ���ʽΪ��Fe2O3+6H��=2 Fe3��+3H2O����2������c=![]() �ã��Ȼ��������ʵ���Ũ��Ϊc=
�ã��Ȼ��������ʵ���Ũ��Ϊc=![]() =6.0mol/L�������Һ�������ӵ����ʵ���Ũ��Ϊ6.0mol/L��3=18.0mol/L��ʵ����û��480mL������ƿ���ʱ�������0.15mol/L��ϡ����500mL�����ƹ���Ϊ�����㡢ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ�ʹ�õ������У���Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ���ȱ��500mL����ƿ������C1V1=C2V2���У�0.15mol/L��0.5L=18.0mol/L��C2��C2=0.0125L=12.5mL������ȡ����Һ�������12.5mL�������ƹ������������ʱ���ӿ̶��ߣ��������������ˮƫ�٣�������ҺŨ��ƫ��ƫ�ߣ���3��������FeCl3��Һ������ڵ�����ˮ�У������Ƶ�Fe��OH��3���壬��Ӧ�Ļ�ѧ��Ӧ����ʽΪ��FeCl3+3 H2O
=6.0mol/L�������Һ�������ӵ����ʵ���Ũ��Ϊ6.0mol/L��3=18.0mol/L��ʵ����û��480mL������ƿ���ʱ�������0.15mol/L��ϡ����500mL�����ƹ���Ϊ�����㡢ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ�ʹ�õ������У���Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ���ȱ��500mL����ƿ������C1V1=C2V2���У�0.15mol/L��0.5L=18.0mol/L��C2��C2=0.0125L=12.5mL������ȡ����Һ�������12.5mL�������ƹ������������ʱ���ӿ̶��ߣ��������������ˮƫ�٣�������ҺŨ��ƫ��ƫ�ߣ���3��������FeCl3��Һ������ڵ�����ˮ�У������Ƶ�Fe��OH��3���壬��Ӧ�Ļ�ѧ��Ӧ����ʽΪ��FeCl3+3 H2O![]() Fe��OH��3�������� +3HCl��A��Fe��OH��3�����DZ����Ȼ����ڷ�ˮ�����ɵľ�һ�ȶ��ķ�ɢϵ����һ�ֺ��ɫ�����塢���Ļ���ѡ��A��ȷ��B�������з�ɢ�ʵ���ֱ����10-9m~10-7m֮�䣬ѡ��B��ȷ��C��������ж����������Һ�����У����ЧӦ����Fe��OH��3�����FeCl3��Һ��������������Fe��OH��3�����FeCl3��Һ��ѡ��C����ȷ��D��ȡ����Fe��OH��3���������Թ��У����Թ�����εμ�ϡ���ᣬ�ȷ�������ľ۳����ʿɿ����Ȳ������ɫ���������������������ϡ���ᣬ�ʳ����ܽ⣬���յõ���ɫ����������Һ��ѡ��D��ȷ��E��Fe��OH��3�������Ӵ�����ɣ��ڵ糡�����·�����Ӿ��������ɵĽ����������ƶ���������������ɫ���ѡ��E����ȷ����ѡCE����4������������Һ�У���μ����Ũ�ȵ�����������Һ��������Ӧ�����ӷ���ʽΪ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O����Һ������Ũ���ȱ�Сֱ��ֻ��ˮ�����ɵij���������������������Ϊ0����������������ʱ�������������������������Ũ������ʹ��Һ������������ǿ��������Һ�ĵ�����������������Һ������Ӷ��仯��ͼ����D��
Fe��OH��3�������� +3HCl��A��Fe��OH��3�����DZ����Ȼ����ڷ�ˮ�����ɵľ�һ�ȶ��ķ�ɢϵ����һ�ֺ��ɫ�����塢���Ļ���ѡ��A��ȷ��B�������з�ɢ�ʵ���ֱ����10-9m~10-7m֮�䣬ѡ��B��ȷ��C��������ж����������Һ�����У����ЧӦ����Fe��OH��3�����FeCl3��Һ��������������Fe��OH��3�����FeCl3��Һ��ѡ��C����ȷ��D��ȡ����Fe��OH��3���������Թ��У����Թ�����εμ�ϡ���ᣬ�ȷ�������ľ۳����ʿɿ����Ȳ������ɫ���������������������ϡ���ᣬ�ʳ����ܽ⣬���յõ���ɫ����������Һ��ѡ��D��ȷ��E��Fe��OH��3�������Ӵ�����ɣ��ڵ糡�����·�����Ӿ��������ɵĽ����������ƶ���������������ɫ���ѡ��E����ȷ����ѡCE����4������������Һ�У���μ����Ũ�ȵ�����������Һ��������Ӧ�����ӷ���ʽΪ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O����Һ������Ũ���ȱ�Сֱ��ֻ��ˮ�����ɵij���������������������Ϊ0����������������ʱ�������������������������Ũ������ʹ��Һ������������ǿ��������Һ�ĵ�����������������Һ������Ӷ��仯��ͼ����D��

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�