��Ŀ����
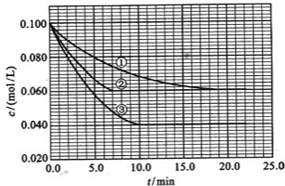
��14�֣�����Һ�У���ӦA+2BC�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�ȷֱ�Ϊc(A)=0.100mol/L��c(B)=0.200mol/L��c(C)=0.000mol/L����Ӧ��AŨ����ʱ��仯��ͼ��ʾ��
��ش��������⣺
��1��ʵ���ƽ��ʱC��Ũ��Ϊ____________��
��2����ٱȽϣ��ں͢۷ֱ���ı�һ�ַ�Ӧ���������У������ı������___________��
�жϵ������ǣ�__________________________________�������ı��������_________��
��3���÷�Ӧ�ġ�H _____0���ж���������___________________________________________��
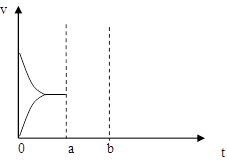
��4����ͼ��ʾʵ��������´ﵽƽ��ʱ�����������ı������Ӧ�ٶȺͻ�ѧƽ��ı仯�����������ʱ����ѷ�Ӧ�ٶȱ仯�������ͼ��a~b����
��12�֣�
��1��0.06mol/L��2�֣�
��2���ڼӴ������ﵽƽ���ʱ�����̣�ƽ��ʱA��Ũ��δ�� ���¶����� ��ÿ��2�֣�
��3�����������¶ȣ�ƽ��ʱAŨ�ȼ�С��ƽ��������Ӧ�����ƶ����ʸ÷�Ӧ�����ȷ�Ӧ��ÿ��2�֣�
��4����2�֣�
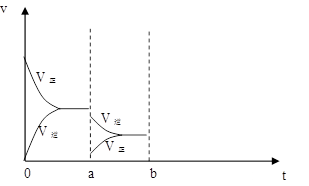
����:��

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
�����Ŀ
 ��2010?����������Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc��A��=0.100mol/L��c��B��=0.200mol/L �� c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ��
��2010?����������Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc��A��=0.100mol/L��c��B��=0.200mol/L �� c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ�� ����Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊ c��A��=1.0mol/L��c��B��=2.0mol/L��c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ����ش��������⣺
����Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊ c��A��=1.0mol/L��c��B��=2.0mol/L��c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ����ش��������⣺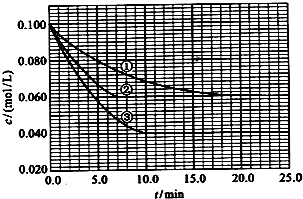 ��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ�
��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ�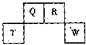 ��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺
��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺