��Ŀ����
�ڿ����ϣ���ʦ��ʾ�˽�������CuSO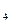 ��Һ�ķ�Ӧ��ͬѧ�ǹ۲쵽�÷�Ӧ����������ɫ��Cu(OH)
��Һ�ķ�Ӧ��ͬѧ�ǹ۲쵽�÷�Ӧ����������ɫ��Cu(OH)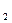 ������û�з���ͭ�������ɡ���ijͬѧ�룺��������ɵ�ͭ���ٱ���ɫ���������ǣ���û�б������أ���������κ�ʵ���Ҽ����о���ϣ����һ����ʵ������֤�Լ��IJ²��Ƿ���ȷ��
������û�з���ͭ�������ɡ���ijͬѧ�룺��������ɵ�ͭ���ٱ���ɫ���������ǣ���û�б������أ���������κ�ʵ���Ҽ����о���ϣ����һ����ʵ������֤�Լ��IJ²��Ƿ���ȷ��
�����ͬѧ���㣬����дһ��ʵ������������ʦ��Ҫ����ʦ�ṩ�������Ʒ��
��1��ʵ��̽��Ŀ�ģ�___________________________��
��2��̽�������ݵĻ�ѧԭ����____________________________��
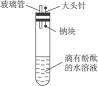
��3��ʵ�����������С��������Ƭ����ֽ��_________��__________��ҩƷ�������ơ�_____��___________����ͬѧ��̽��ʵ��������ط������ɵ���ɫ�����л��������ĺ�ɫ���������ʹ�õ�ҩƷ��û�����⣬����Ϊ�ú�ɫ��������___________�����ɸú�ɫ�������ԭ����_______________________________________________��
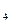 ��Һ�ķ�Ӧ��ͬѧ�ǹ۲쵽�÷�Ӧ����������ɫ��Cu(OH)
��Һ�ķ�Ӧ��ͬѧ�ǹ۲쵽�÷�Ӧ����������ɫ��Cu(OH)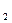 ������û�з���ͭ�������ɡ���ijͬѧ�룺��������ɵ�ͭ���ٱ���ɫ���������ǣ���û�б������أ���������κ�ʵ���Ҽ����о���ϣ����һ����ʵ������֤�Լ��IJ²��Ƿ���ȷ��
������û�з���ͭ�������ɡ���ijͬѧ�룺��������ɵ�ͭ���ٱ���ɫ���������ǣ���û�б������أ���������κ�ʵ���Ҽ����о���ϣ����һ����ʵ������֤�Լ��IJ²��Ƿ���ȷ�������ͬѧ���㣬����дһ��ʵ������������ʦ��Ҫ����ʦ�ṩ�������Ʒ��
��1��ʵ��̽��Ŀ�ģ�___________________________��
��2��̽�������ݵĻ�ѧԭ����____________________________��
��3��ʵ�����������С��������Ƭ����ֽ��_________��__________��ҩƷ�������ơ�_____��___________����ͬѧ��̽��ʵ��������ط������ɵ���ɫ�����л��������ĺ�ɫ���������ʹ�õ�ҩƷ��û�����⣬����Ϊ�ú�ɫ��������___________�����ɸú�ɫ�������ԭ����_______________________________________________��
��1��ȷ������CuSO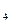 ��Һ��Ӧ�Ƿ���ͭ����
��Һ��Ӧ�Ƿ���ͭ����
��2��������ͭ�����������ͭ���ܡ�Cu(OH)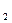 +2HCl
+2HCl CuCl
CuCl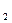 +2H
+2H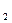 O��
O��
(3)�����ձ�CuSO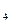 ��Һ���ᣨ��ϡ���ᣩCuO ����ˮ��Ӧ�ų�����ʹ���ɵ�Cu(OH)
��Һ���ᣨ��ϡ���ᣩCuO ����ˮ��Ӧ�ų�����ʹ���ɵ�Cu(OH)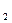 ���ַֽ⣺Cu(OH)
���ַֽ⣺Cu(OH)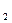
 CuO+H
CuO+H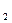 O
O
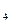 ��Һ��Ӧ�Ƿ���ͭ����
��Һ��Ӧ�Ƿ���ͭ������2��������ͭ�����������ͭ���ܡ�Cu(OH)
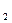 +2HCl
+2HCl CuCl
CuCl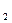 +2H
+2H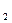 O��
O��(3)�����ձ�CuSO
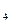 ��Һ���ᣨ��ϡ���ᣩCuO ����ˮ��Ӧ�ų�����ʹ���ɵ�Cu(OH)
��Һ���ᣨ��ϡ���ᣩCuO ����ˮ��Ӧ�ų�����ʹ���ɵ�Cu(OH)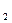 ���ַֽ⣺Cu(OH)
���ַֽ⣺Cu(OH)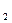
 CuO+H
CuO+H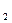 O
O��̽������CuSO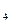 ��Һ��Ӧ�Ƿ��û���ͭ��Ӧ��Cu��Cu(OH)
��Һ��Ӧ�Ƿ��û���ͭ��Ӧ��Cu��Cu(OH)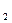 �IJ�ͬ�������֣����ʵ���ǽ���������������������ܽ⣬���˷�����ȷ�������������ҩƷ��
�IJ�ͬ�������֣����ʵ���ǽ���������������������ܽ⣬���˷�����ȷ�������������ҩƷ��
���ں�ɫ�����ֻ������CuO������ͱ���Cu(OH)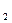 �ֽ��ˡ�ʵ���ϣ���ʵ�������ɢ�Ȳ��죬�����׳��ִ�����
�ֽ��ˡ�ʵ���ϣ���ʵ�������ɢ�Ȳ��죬�����׳��ִ�����
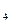 ��Һ��Ӧ�Ƿ��û���ͭ��Ӧ��Cu��Cu(OH)
��Һ��Ӧ�Ƿ��û���ͭ��Ӧ��Cu��Cu(OH) �IJ�ͬ�������֣����ʵ���ǽ���������������������ܽ⣬���˷�����ȷ�������������ҩƷ��
�IJ�ͬ�������֣����ʵ���ǽ���������������������ܽ⣬���˷�����ȷ�������������ҩƷ�����ں�ɫ�����ֻ������CuO������ͱ���Cu(OH)
 �ֽ��ˡ�ʵ���ϣ���ʵ�������ɢ�Ȳ��죬�����׳��ִ�����
�ֽ��ˡ�ʵ���ϣ���ʵ�������ɢ�Ȳ��죬�����׳��ִ�����
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 +2H+====H2O+CO2��
+2H+====H2O+CO2��