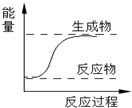
��Ŀ����
 ��ش��ȼ���������⣺
��ش��ȼ���������⣺ ��1���������ռ��ǵ��ʳ��ˮʱ���չ̶��ı���k�������ȣ����ɵIJ�Ʒ��������k��_______��Ҫ��������ʽ�ͽ����;
��1���������ռ��ǵ��ʳ��ˮʱ���չ̶��ı���k�������ȣ����ɵIJ�Ʒ��������k��_______��Ҫ��������ʽ�ͽ����; ��2��ԭ�ϴ����г�������ɳ��Ca2����Mg2����Fe3����SO42�������ʣ����뾫�ƺ���ܹ����ʹ�á�����ʱ����������ˮ���˺�Ҫ������Լ��ֱ�Ϊ��Na2CO3����HCl�����ᣩ��BaCl2����3���Լ����ӵĺ���˳����______________������ţ�
��2��ԭ�ϴ����г�������ɳ��Ca2����Mg2����Fe3����SO42�������ʣ����뾫�ƺ���ܹ����ʹ�á�����ʱ����������ˮ���˺�Ҫ������Լ��ֱ�Ϊ��Na2CO3����HCl�����ᣩ��BaCl2����3���Լ����ӵĺ���˳����______________������ţ� ��3���ȼҵ�Ǹߺ��ܲ�ҵ��һ�ֽ�������ȼ�ϵ������ϵ��¹��տ��Խڣ��磩��30�����ϡ������ֹ�������У�������ϵĴ�����ת����ϵ����ͼ��ʾ�����еĵ缫δ��������õ�����Ĥ��ֻ����������ͨ����
��3���ȼҵ�Ǹߺ��ܲ�ҵ��һ�ֽ�������ȼ�ϵ������ϵ��¹��տ��Խڣ��磩��30�����ϡ������ֹ�������У�������ϵĴ�����ת����ϵ����ͼ��ʾ�����еĵ缫δ��������õ�����Ĥ��ֻ����������ͨ���� ��
��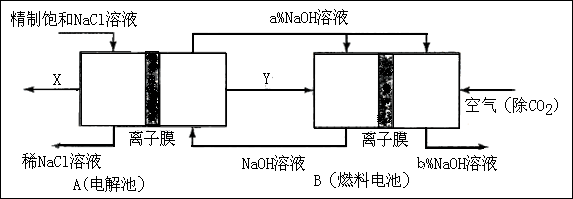 ͼ��X��Y�ֱ���_____��_______���ѧʽ���������Ƚ�ͼʾ������������������a����b���Ĵ�С_________;
ͼ��X��Y�ֱ���_____��_______���ѧʽ���������Ƚ�ͼʾ������������������a����b���Ĵ�С_________; �ڷֱ�д��ȼ�ϵ��B�������������Ϸ����ĵ缫��Ӧ������______; ������_____;
�ڷֱ�д��ȼ�ϵ��B�������������Ϸ����ĵ缫��Ӧ������______; ������_____; ��������Ƶ���Ҫ�ڣ��磩��֮�����ڣ�д��2����____________��____________��
��������Ƶ���Ҫ�ڣ��磩��֮�����ڣ�д��2����____________��____________����1��k=M(Cl2)/2 M(NaOH)=71/80=1:1.13��0.89
��2���ۢ٢�
��3����Cl2 H2 a��С��b�� ��O2+4e-+2H2O��4OH- H2��2e-+2OH-��2H2O ��ȼ�ϵ�ؿ��Բ���������ĵĵ��ܣ���߲�����Һ��Ũ�ȣ������ܺ�
������������Ҳ���֣�
��2���ۢ٢�
��3����Cl2 H2 a��С��b�� ��O2+4e-+2H2O��4OH- H2��2e-+2OH-��2H2O ��ȼ�ϵ�ؿ��Բ���������ĵĵ��ܣ���߲�����Һ��Ũ�ȣ������ܺ�
������������Ҳ���֣�
��1��ֻҪ�˽��ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ2NaCl+2H2O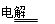 2NaOH+H2��+Cl2�����ɵõ���k=M(Cl2)/2 M(NaOH)=71/80=1:1.13��0.89��
2NaOH+H2��+Cl2�����ɵõ���k=M(Cl2)/2 M(NaOH)=71/80=1:1.13��0.89��
��2��ֻҪץס��������Ҫ���ڳ�̼�������ǰ���ɵõ�˳���ϵ���ۢ٢ڣ�
��3������ͻ�ƿ�����Bȼ�ϵ����ߣ�ͨ����һ��Ϊ��������ԭ��Ӧ������ô��߱�ȻͨH2������Y��ΪH2����ת��������ݵ�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ��֪Ψһδ֪�ľ���X����ȻΪCl2�ˣ�A�е�NaOH����ȼ�ϵ�������ٳ���������O2+4e-+2H2O��4OH- ��֪NaOH+Ũ������
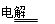 2NaOH+H2��+Cl2�����ɵõ���k=M(Cl2)/2 M(NaOH)=71/80=1:1.13��0.89��
2NaOH+H2��+Cl2�����ɵõ���k=M(Cl2)/2 M(NaOH)=71/80=1:1.13��0.89����2��ֻҪץס��������Ҫ���ڳ�̼�������ǰ���ɵõ�˳���ϵ���ۢ٢ڣ�
��3������ͻ�ƿ�����Bȼ�ϵ����ߣ�ͨ����һ��Ϊ��������ԭ��Ӧ������ô��߱�ȻͨH2������Y��ΪH2����ת��������ݵ�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ��֪Ψһδ֪�ľ���X����ȻΪCl2�ˣ�A�е�NaOH����ȼ�ϵ�������ٳ���������O2+4e-+2H2O��4OH- ��֪NaOH+Ũ������

��ϰ��ϵ�д�
�����Ŀ
 NH4++OH-������N2H4���������ᷴӦ�Ļ�ѧ����ʽ�� ��
NH4++OH-������N2H4���������ᷴӦ�Ļ�ѧ����ʽ�� ��