��Ŀ����
��֪A��B��C��D��EΪ��ѧ��ѧ�еij���������ס��ҡ���Ϊ�������еķǽ������ʣ���Ϊ�������ʣ�C����ɫ��Ӧ�ʻ�ɫ�������ȼҵ����Ҫ�������֮����ת����ϵ���£��еķ�Ӧ���ֲ����Ѿ���ȥ��
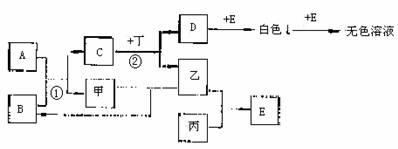
��1���Ļ�ѧʽΪ ��
��2���������ڱ��е�λ��Ϊ ��
��3����Ӧ�١��ڵĻ�ѧ����ʽ�ֱ�Ϊ��
��Ӧ�� �� ��Ӧ�� ��
��4��д��C������Ӧ�����ӷ���ʽ ��
��1��O2
��2����������VIIA��
��3��2Na2O2+2H2O=4aOH+O2�� 2A1+2NaOH+2H2O=2NaA1O2+3H2��
��4��C13+2OH-=C1O-+C1-+H2O

��ϰ��ϵ�д�
�����Ŀ
��֪A��B��C��D�ֱ���Cu��Ag��Fe��Al���ֽ����е�һ�֣���֪��A��C������ϡ���ᷴӦ�ų����壻��B��D�������η�Ӧ���û�������D����C��ǿ�Ӧ�ų����壬�ɴ˿����ƶ�A��B��C��D�����ǣ�������
| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |

 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�