��Ŀ����
��6�֣�����һ��ɫ�Ļ�����ˮ��Һ��ֻ���ܺ������������е������֣�K����Al3����Fe3����Mg2����Ba2���� ��Cl��
��Cl��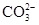 ��
�� ����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
(1)һ�������ڵ�������________________
(2)����ȷ���Ƿ���ڵ�������
(3)��ȷ��K���Ƿ����________(��ǡ���)���жϵ�������
 ��Cl��
��Cl��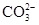 ��
�� ����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�(1)һ�������ڵ�������________________
(2)����ȷ���Ƿ���ڵ�������
(3)��ȷ��K���Ƿ����________(��ǡ���)���жϵ�������
��6��,ÿС��2�֣�
(1)Fe3+ Mg2+ Ba2+ CO32- (2)Cl��
(3) �ǡ���Һ�п϶����ڵ������� ��Al3����SO42-�������㣬
��Al3����SO42-�������㣬 ��Al3�����ʵ�������0.02 mol��SO42-���ʵ�����0.05 mol..���ݵ���غ㣬K��һ������
��Al3�����ʵ�������0.02 mol��SO42-���ʵ�����0.05 mol..���ݵ���غ㣬K��һ������
(1)Fe3+ Mg2+ Ba2+ CO32- (2)Cl��
(3) �ǡ���Һ�п϶����ڵ�������
 ��Al3����SO42-�������㣬
��Al3����SO42-�������㣬 ��Al3�����ʵ�������0.02 mol��SO42-���ʵ�����0.05 mol..���ݵ���غ㣬K��һ������
��Al3�����ʵ�������0.02 mol��SO42-���ʵ�����0.05 mol..���ݵ���غ㣬K��һ������ԭ��Һ��ɫ�����ų�Fe3���Ĵ��ڣ�
�������NaOH��Һ���Ⱥ��ռ���������ض�Ϊ������NH4����OH�� NH3����H2O�������ʵ���Ϊ0.02mol��ͬʱ��������˵��ԭ��Һ�в����ܺ���Mg2+
NH3����H2O�������ʵ���Ϊ0.02mol��ͬʱ��������˵��ԭ��Һ�в����ܺ���Mg2+
����Һ��ͨ�����CO2�����ɰ�ɫ���������֪ԭ��Һ�к���Al3����Al3+4OH��=AlO2����2H2O��AlO2����CO2��2H2O=Al(OH)3��+HCO3����2Al(OH)3 Al2O3��3H2O����Al2O3������Ϊ1.02 g���ɵ�ԭ��Һ��Al3�������ʵ���Ϊ0.02mol������Al3���Ĵ��ڣ�ͬʱ�ɷ���CO32-�Ĵ��ڣ�˫ˮ�ⷴӦ����
Al2O3��3H2O����Al2O3������Ϊ1.02 g���ɵ�ԭ��Һ��Al3�������ʵ���Ϊ0.02mol������Al3���Ĵ��ڣ�ͬʱ�ɷ���CO32-�Ĵ��ڣ�˫ˮ�ⷴӦ����
ԭ��Һ�м�����BaCl2��Һ�ð�ɫ�����Ҳ��������ᣬ��֪ԭ��Һ�к���SO42-(ͬʱҲ���ų���Ba2+���ڵĿ�����)��SO42����Ba2��=BaSO4����������Ϊ11.65 g�������ԭ��Һ�е�SO42-���ʵ�����0.05 mol��
�ۺ�������Ϣ���Ծɲ���ȷ���Ƿ���ڵ�������Cl�������ݵ���غ��֪�ض�����K��
�������NaOH��Һ���Ⱥ��ռ���������ض�Ϊ������NH4����OH��
 NH3����H2O�������ʵ���Ϊ0.02mol��ͬʱ��������˵��ԭ��Һ�в����ܺ���Mg2+
NH3����H2O�������ʵ���Ϊ0.02mol��ͬʱ��������˵��ԭ��Һ�в����ܺ���Mg2+����Һ��ͨ�����CO2�����ɰ�ɫ���������֪ԭ��Һ�к���Al3����Al3+4OH��=AlO2����2H2O��AlO2����CO2��2H2O=Al(OH)3��+HCO3����2Al(OH)3
 Al2O3��3H2O����Al2O3������Ϊ1.02 g���ɵ�ԭ��Һ��Al3�������ʵ���Ϊ0.02mol������Al3���Ĵ��ڣ�ͬʱ�ɷ���CO32-�Ĵ��ڣ�˫ˮ�ⷴӦ����
Al2O3��3H2O����Al2O3������Ϊ1.02 g���ɵ�ԭ��Һ��Al3�������ʵ���Ϊ0.02mol������Al3���Ĵ��ڣ�ͬʱ�ɷ���CO32-�Ĵ��ڣ�˫ˮ�ⷴӦ����ԭ��Һ�м�����BaCl2��Һ�ð�ɫ�����Ҳ��������ᣬ��֪ԭ��Һ�к���SO42-(ͬʱҲ���ų���Ba2+���ڵĿ�����)��SO42����Ba2��=BaSO4����������Ϊ11.65 g�������ԭ��Һ�е�SO42-���ʵ�����0.05 mol��
�ۺ�������Ϣ���Ծɲ���ȷ���Ƿ���ڵ�������Cl�������ݵ���غ��֪�ض�����K��

��ϰ��ϵ�д�
�����Ŀ
 Fe3O4��4H2
Fe3O4��4H2