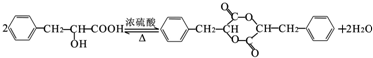
��Ŀ����
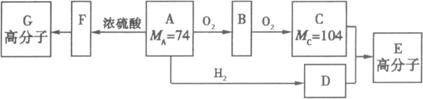
(8��)��һ�������¿�ʵ����ͼ��ʾ����֮��ı仯: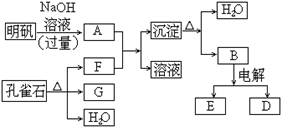
����д���¿հ�:
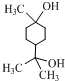
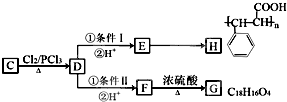
��1����ȸʯ����Ҫ�ɷ���Cu2(OH)2CO3�������ֽ⡣��ͼ�е�F�ĵ���ʽΪ ��
��2��д��������Һ�����NaOH��Һ��Ӧ�����ӷ���ʽ ��
��3��ͼ������G��D��Ϊ���壬��ͺ��ڸ����¿ɷ�����Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4��D��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ���õ����ű������ת�Ƶķ�����Ŀ�� ��
��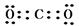 ��Al3++4OH-=AlO2-+2H2O;
��Al3++4OH-=AlO2-+2H2O;
��3CuO +2Al 3Cu+Al2O3
3Cu+Al2O3
��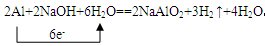
����

��ϰ��ϵ�д�
�����Ŀ
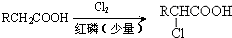
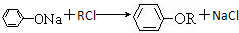
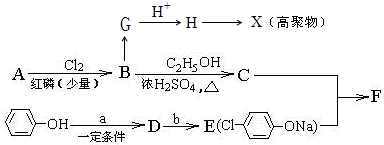
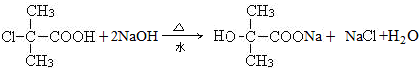
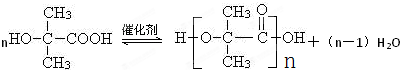
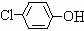
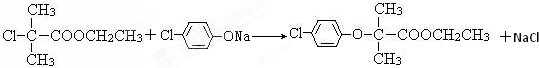
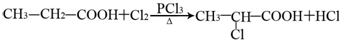
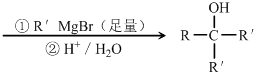
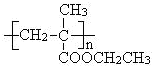 ������Ӧ�Ļ�ѧ����ʽ�ǣ���һ����
������Ӧ�Ļ�ѧ����ʽ�ǣ���һ����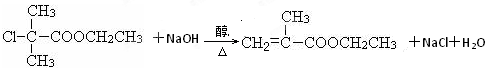

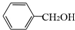
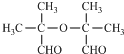
 �ṹ����
�ṹ����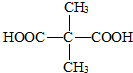
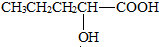
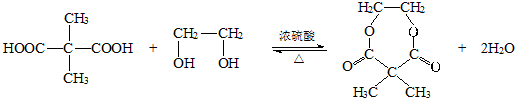
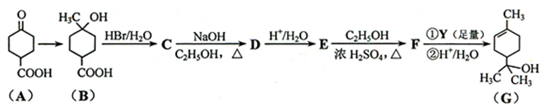
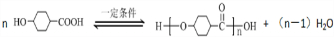
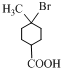 +2NaOH
+2NaOH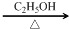
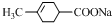 +NaBr+2H2O
+NaBr+2H2O