��Ŀ����
����Ŀ�������Ȼ�ѧ����ʽ��д��ȷ����
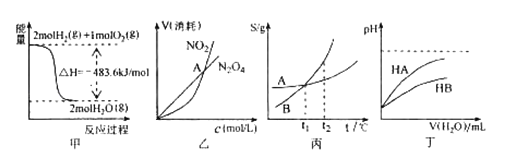
A. C2H5OH(l)��3O2(g)===2CO2(g)��3H2O(g) ��H=��1 367.0 kJ��mol��1
B. NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l) ��H=��57.3 kJ��mol��1
C. S(s)��O2(g)===SO2(g) ��H=��269.8 kJ��mol��1
D. 2NO2===O2��2NO ��H=��116.2 kJ��mol��1
���𰸡�C
��������
A���Ҵ�ȼ���Ƿ��ȷ�Ӧ����H��0��A����
B���кͷ�Ӧ�Ƿ��ȷ�Ӧ����H��0��B����
C����ȼ�շ��ȣ��Ȼ�ѧ����ʽ��ȷ��C��ȷ��
D��û��ע�����ʵ�״̬��D����
��ѡC��

��ϰ��ϵ�д�
�����Ŀ