��Ŀ����
����Ŀ����п��������������ηɻ��������״ﲨͿ�ϡ����ԷϾ�п�̵��Ϊԭ���Ʊ���п���������Ҫ�������£�

(1)����п�̸ɵ�أ������Ϊ KOH���� MnO2���뷴Ӧ�ĵ缫��ӦʽΪ______________________��
(2)���������ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ��________________________����дһ�㣩�����ʱ���������̱�˫��ˮ��ԭ�Ļ�ѧ����ʽΪ___________________��
(3)�������۳���ʱ�����۵�������______________ (��������� ��ԭ���� ���������� �� ��
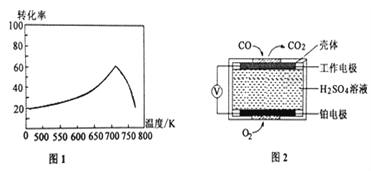
(4)�������Ե���Ϊ����������Һ������������ KMnO4��Һ�������ն�ʵ�ֵġ� �ڲ�ͬpH�£�KMnO4��Һ��Hg�������ʵ�Ӱ�켰��Ҫ������ͼ��ʾ��

������ݸ�ͼ���� pH�Թ�������Ӱ��ı仯���ɣ� __________________________��
���Է�����ǿ���Ի����� Hg �ĵ�λʱ�������ʸߵ�ԭ������ǣ� _______________��
(5)���ⶨ��Һ�ɷֺ�ͬʱ����һ������ MnSO4�����۵�Ŀ����________________��
(6)�� x��0.2 ʱ�����õ�����п��������״ﲨ�����������ر�ǿ���������������ʽ��ʾ�����������_________��
���𰸡� MnO2 +e-+H2O=MnOOH+OH- ���ȡ����衢��������Ũ�ȵ� MnO2 + H2O2 + H2SO4��MnSO4 +O2�� + 2H2O ��ԭ�� �� pH�����߹����������Ƚ��ͺ����� Mn2+���д����ã���λʱ���������ʸ� ������Һ���ӵijɷ֣�����ˮ�Ⱥ�����п����������� MnO��4ZnO��5Fe2O3
��������(1)����п�̸ɵ�أ������Ϊ KOH���� MnO2��Ϊ�������ϲ��õ��Ӳ���MnOOH���䷴Ӧ�ĵ缫��ӦʽΪ��MnO2 +e-+H2O=MnOOH+OH-����2��Ӱ�컯ѧ��Ӧ���ʵ��������¶ȡ���Ӧ�����ȡ��Ӵ������ѹǿ�ȣ��ʽ������ʵ�ʣ����������ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�У����ȡ����衢��������Ũ�ȵȣ��������̿�֪�����ʱ���������̱�˫��ˮ��ԭ���������̣�˫��ˮ�����������������䷴Ӧ�Ļ�ѧ����ʽΪ��MnO2 + H2O2 + H2SO4��MnSO4 +O2�� + 2H2O��(3)���ǻ��õĽ��������Ի������۳���ʱ�����۵������ǻ�ԭ������4���ٸ���ͼ���֪������pH�����߹���ȥ�����Ƚ��ͺ����ӣ��ڸ���ͼ���֪����ǿ���������£�MnO4���Ļ�ԭ������Mn2����Mn2+���д����ã����Ե�λʱ���������ʸߣ���5��������п����������ɿ�֪������һ������MnSO4�����ۿ��Ե�����Һ���ӵijɷ֣�ʹ�����ˮ�Ⱥ�����п����������ɣ���6����x��0.2ʱ�������ʵĻ�ѧʽ�ɱ�ʾΪMn02Zn08Fe2O4����Mn��Zn��Fe��ԭ�Ӹ���֮�ȣ�1:4:10����������������ʽ�ɱ�ʾΪMnO4ZnO5Fe2O3��

����Ŀ����.���������ɡ��ܽ��ת���������Ʊ����ᴿ�Լ����е������й㷺Ӧ�á�
��1����֪25��ʱ��Ksp(BaSO4)��1��10��10����BaSO4������Һ��������Һ��c(Ba2��)��_______mol��L��1��ȡ100 mL��Һ��100 mL 2 mol��L��1��Na2SO4��Һ��ϣ����Һ��c(Ba2��)��___________ mol��L��1��
��2������ʹ�õĹ�¯��Ҫ���ڳ�ˮ��������ή��ȼ�ϵ������ʡ�ˮ���к��е�CaSO4��������Na2CO3��Һ������ʹ֮ת��Ϊ���ɡ����������CaCO3�����������ȥ��
��CaSO4ת��ΪCaCO3�����ӷ���ʽΪ��_____________________________________________________
�������CaSO4ת��ΪCaCO3��ԭ����______________________________________________________
��.��25 ��ʱ��HSCN��HClO��H2CO3�ĵ��볣�����±���
HClO | HSCN | H2CO3 |
K��3.2��10��8 | K��0.13 | K1��4.2��10��7 K2��5.6��10��11 |
��1��1 mol��L��1��KSCN��Һ�У��������ӵ�Ũ���ɴ�С��˳��Ϊ_________________________________
��2����Na2CO3��Һ�м������HClO��Һ����Ӧ�Ļ�ѧ����ʽΪ_______________________________
��3��25 ��ʱ��Ϊ֤��HClOΪ���ᣬijѧϰС���ͬѧ�������������ʵ�鷽�����������ַ����У�����Ϊ�ܹ��ﵽʵ��Ŀ�ĵ���______________________(�����и��������)��
a����pH�Ʋ���0.1mol��L��1NaClO��Һ��pH�������pH>7����֤��HClOΪ����
b����pH��ֽ����0.01 mol��L��1 HClO��Һ��pH�������pH>2����֤��HClOΪ����
c������������Ũ�Ⱦ�Ϊ0.1 mol��L��1��HClO��Һ������ĵ����ԣ������HClO��Һ�ĵ������������ᣬ��֤��HClOΪ����
����Ŀ��ijС��ͬѧ����ԭ���װ��̽�����ʵ����ʡ�
������ʾ��ԭ���װ���У�������Ӧ��Ļ�ԭ��Խǿ����������Ӧ���������Խǿ��ԭ��صĵ�ѹԽ�� |
��1��ͬѧ�������±���װ�ý���ʵ�鲢��¼��
װ�� | ��� | �缫A | ��ҺB | ���������� |
| �� | Fe | pH=2�� H2SO4 | ����װ�ú�ʯī���������ɫ���ݣ���ѹ��ָ��ƫת |
�� | Cu | pH=2��H2SO4 | ����װ�ú�ʯī��������������ѹ��ָ��ƫת����¼����Ϊa |
��ͬѧ����Ϊʵ����������Ҫ���������ⸯʴ����������Ӧʽ��_________��
�����ʵ��������ͬѧ��Ϊ�����ܷ������ⸯʴ�����ж�������_________����ͬѧ��Ϊʵ������Ӧ����������ʴ���������ĵ缫��Ӧʽ��_________��
��2��ͬѧ����������װ�ò���Cu��ʯīΪ�缫����ʵ�飬̽��ʵ����ָ��ƫתԭ��Ӱ��O2�����Ե����ء�
��� | ��ҺB | ���������� |
�� | ����е�pH=2�� H2SO4 | ��Һ������ú���ǣ�����װ�ú�ѹ��ָ��ƫת����¼����Ϊb |
�� | pH=2��H2SO4 | ��ʯīһ���ͨ��O2������װ�ã���ѹ��ָ��ƫת����¼����Ϊc��ȡ���缫������Һ�м�������ŨNa2SO4��Һ��Ϻ���缫������O2ͨ�룬��ѹ��������Ϊc |
�� | pH=12��NaOH | ��ʯīһ���ͨ��O2������װ�ã���ѹ��ָ��ƫת����¼����Ϊd |
����ͬѧ�Ƚ�ʵ�������������ĵ�ѹ������Ϊ��c��a��b�������ԭ����_________��
����ͬѧ�����������бȽϣ���Ŀ����̽����O2�����Ե�_________Ӱ�졣
��ʵ�����м���Na2SO4��Һ��Ŀ����_________��
��Ϊ�ﵽ��ͬѧ��Ŀ�ģ������ۣ�ͬѧ����ΪӦ������ͼװ�ö��������ظ�����ʵ�飬�������ͼ��_________���ظ�ʵ��ʱ����¼��ѹ����������Ϊc����d������c����d�����ɴ˵ó��Ľ�����_________��