��Ŀ����
��12�֣��̶�������CO2����Ч��������Դ�������ٿ����е��������壮CO2�뻯����A��Ӧ���ɻ�����B�����·�Ӧʽ�������Լ������P��Ӧ������ʡ�ԣ���

��1��������B�ķ���ʽΪ__________��1 mol��������ȫȼ��������_________mol O2 ��
��2���� ͨ����ȥ��Ӧ�Ʊ�A�Ļ�ѧ����ʽΪ(ע����Ӧ����)��
ͨ����ȥ��Ӧ�Ʊ�A�Ļ�ѧ����ʽΪ(ע����Ӧ����)��
_______________________________________________________________________��
��3��B�����C2H5OH������·���������Ӧ�����ɵ��л���Ľṹ��ʽΪ��
__________________________________________��
��4����CO2���ƣ�COҲ�ܱ��̶������á���һ�������£�CO��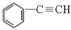 ��
��
H2���߷�����Ӧ�����������뷴Ӧ�������ɻ�����C�ķ���ʽΪC9H8O��������C�ܷ���������Ӧ��д��������C���ܵĽṹ��ʽ��
___________________________________________________________________��
���𰸡�
��1�� C10H10O4 ��10.5
��2��
��3��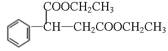
��4��
��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 ͨ����ȥ��Ӧ�Ʊ���Ļ�ѧ����ʽΪ
ͨ����ȥ��Ӧ�Ʊ���Ļ�ѧ����ʽΪ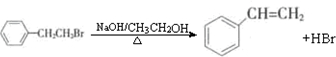

 ����дһ�֣�
����дһ�֣� ��H2���߷�����Ӧ�����������뷴Ӧ�������ɻ�������͢��������ʽ��ΪC9H8O���Ҷ��ܷ���������Ӧ�����й��ڢ��͢���˵����ȷ����
��H2���߷�����Ӧ�����������뷴Ӧ�������ɻ�������͢��������ʽ��ΪC9H8O���Ҷ��ܷ���������Ӧ�����й��ڢ��͢���˵����ȷ����
 �̶�������CO2����Ч��������Դ�������ٿ����е��������壮��ҵ����һ����CO2�������״�ȼ�ϵķ�����
�̶�������CO2����Ч��������Դ�������ٿ����е��������壮��ҵ����һ����CO2�������״�ȼ�ϵķ����� CH3OH��g��+H2O��g��+49kJ
CH3OH��g��+H2O��g��+49kJ
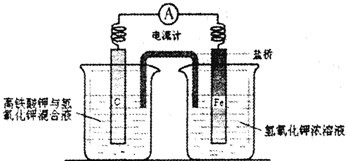
 CH3OH(g)��H2O(g) ��H ��
��49.0 kJ��mol��1��
CH3OH(g)��H2O(g) ��H ��
��49.0 kJ��mol��1��