��Ŀ����
��16�֣�X��Y��Z��W�Ƕ����ڵ�����Ԫ�أ��й����ǵ���Ϣ���±���ʾ��
| Ԫ�� | ���ֽṹ֪ʶ | �������� |
| X | X�ĵ�����˫ԭ�ӷ��ӹ��ɣ���������14������ | X�ж����������XO��XO2��X2O4�ȣ�ͨ�������XO2��X2O4���� |
| Y | Yԭ�ӵĴ�������������������������һ�� | Y���γɶ�����̬�⻯�� |
| Z | Zԭ�ӵ���������������4 | Z����������ϼ���������ϼ۴�����Ϊ6 |
| W | Wԭ�ӵ���������������2n-3��nΪԭ�Ӻ�����Ӳ����� | ��ѧ��Ӧ��Wԭ����ʧȥ���������γ�Wn+ |
��1��X����̬�⻯����ӵĵ���ʽ�� ��ZԪ�������ڱ��е�λ���� ��
��2��X��Y��Z��Ԫ�ص�����������ˮ����������ǿ������˳���� ��
��3������ʱ��W����������Һ��pH 7(��������������������������ǣ� �������ӷ���ʽ��ʾ����
��4��ʵ������X���⻯���ˮ��Һ��ȡW����������ķ����ǣ������ӷ���ʽ��ʾ��
��
��5��25�桢101 kPaʱ��32 g Y����ͼ���̬�⻯����ȫȼ�������ȶ���������ʱ�ų�1780.6 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��1��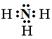 ��2�֣� �����ڣ���A�壨2�֣�
��2�֣� �����ڣ���A�壨2�֣�
��2��HClO4��HNO3��H2CO3 ��2�֣�
��3���� ��2�֣� Al3++3H2O Al(OH)3+3H+��2�֣�
Al(OH)3+3H+��2�֣�
��4��Al3++3NH3��H2O == Al(OH)3��+3NH4+��3�֣�
��5��CH4(g)+2O2(g) == CO2(g)+2H2O(l) ��H��-890.3 kJ/mol ��3�֣�
����

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
�����Ŀ
X��Y��Z��WΪ����������Ԫ�أ�ԭ��������������X������������������2����Y����̬�⻯�������������ˮ������γ��Σ�Z�������������������������Ϊ3��8������˵����ȷ���ǣ�������
| A��X��Y��W����ۺ���������˳��Y��W��X | B����̬�⻯����ȶ��ԣ�Z��W | C��X��W�γɵĻ�����XW4�������ӻ����� | D��ԭ�Ӱ뾶��Z��W��X��Y |

