��Ŀ����
һ�������·�����Ӧ��M2(?)+2R2(g) M2R4(g)
M2R4(g)
(1)���ݻ�һ�����¶�һ�����ܱ������м���һ����M2��R2����Ӧ�����в�������ƽ����Է���������ʱ��ı仯����ͼa��ʾ�����M2���ʵ�״̬�ж���ȷ����______(�����)
A��ֻ��Ϊ���� B��ֻ��Ϊ���� C���ȿ���Ϊ���壬Ҳ����Ϊ����
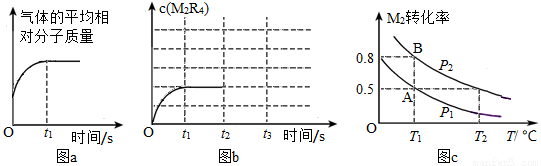
(2)����t2ʱ��ά���¶Ȳ��佫�������Ѹ��ѹ��Ϊԭ����һ�룬t3ʱ�ﵽ�µ�ƽ�⡣����ͼb�л���t2��c(M2R4)�ı仯���ߡ�
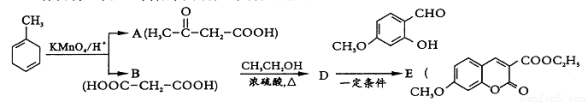
(3)��M2Ϊ��̬��������ͼc�ش��������⣺
�ٸ÷�ӦΪ________(��š�������)�ȷ�Ӧ��P2________P1(���������)��
����һ�ݻ��ɱ���ܱ������г���10mo1M2�����30mo1R2���壬���ﵽƽ��״̬Aʱ�������ݻ�Ϊ20L���練Ӧ��ʼʱ�Գ���10mo1M2��30mo1R2������ƽ��״̬Bʱ�������ݻ�V (B)=____________L��
���ڼס��ҡ���������ͬ�ܱ������а���ͬ��ʽͶ�ϣ�һ�������·����÷�Ӧ(��ʼ�¶Ⱥ���ʼ�����ݻ�����ͬ)���й��������±���ʾ��
���� | �� | �� | �� |
������� | ���º��� | ���Ⱥ��� | ���º�ѹ |
��Ӧ��Ͷ�� | 1 mol M2��2 mol R2 | 1 mol M2R4 | 1 mol M2R4 |
ƽ��ʱ������� | V (��) | V (��) | V (��) |
��Ӧ��ƽ�ⳣ��K | K(��) | K(��) | K(��) |
ƽ��ʱM2R4��Ũ��(mol��L-1) | c (��) | c (��) | c(��) |
ƽ��ʱM2R4�ķ�Ӧ����(mol��L-1��min-1) | v (��) | v (��) | v (��) |
������˵����ȷ����_________��
A��V (��)��V (��) B��K(��)��K(��) C��c (��)��c (��) D��v (��)=v (��)


 �Ĺ���������Ϊ____________��
�Ĺ���������Ϊ____________��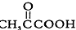 ��ϵͳ����Ϊ2-��ͪ�ᣬ��A�������� ��
��ϵͳ����Ϊ2-��ͪ�ᣬ��A�������� ��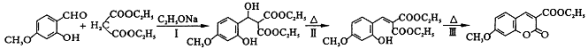
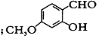 ������ϡNaOH��Һ��Ӧ�Ĺ������� ������ù����ŵķ�����_________��
������ϡNaOH��Һ��Ӧ�Ĺ������� ������ù����ŵķ�����_________��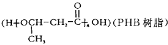 ��д����Ӧ�ϳ���·______________�������Լ����ã�
��д����Ӧ�ϳ���·______________�������Լ����ã�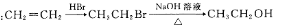
 ����ͭ�������ϡ�����У��õ�SnCl2��PbCl2�Ļ����Һ���ռ�����������101kPa�£���ȴ��25����272mL����ȴ��10����267mL����֪����ͭ�Ͻ���ͭ������������84.8%���������ͭ�Ͻ�������Ǧ������������
����ͭ�������ϡ�����У��õ�SnCl2��PbCl2�Ļ����Һ���ռ�����������101kPa�£���ȴ��25����272mL����ȴ��10����267mL����֪����ͭ�Ͻ���ͭ������������84.8%���������ͭ�Ͻ�������Ǧ������������