��Ŀ����
����Ԫ�����ڱ���ǰ20������Ԫ�أ�A��B��C��D��E��A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��0.9g D�ĵ��ʸ��������ᷴӦ������D3+��1.12L�������������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��
��1��д����Ԫ�ط��ţ�
A______��B______��C______��D______��E______��
��2��B��D������������Ӧˮ�������Ӧ�����ӷ���ʽΪ��______��
��3���õ���ʽ��ʾC��E�γ�E2C�Ĺ��̣�______��
��1��д����Ԫ�ط��ţ�
A______��B______��C______��D______��E______��
��2��B��D������������Ӧˮ�������Ӧ�����ӷ���ʽΪ��______��
��3���õ���ʽ��ʾC��E�γ�E2C�Ĺ��̣�______��
����Ԫ�����ڱ���ǰ20������Ԫ�أ�A��B��C��D��E��A��ԭ�Ӻ���û�����ӣ���AΪ��Ԫ�أ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5����B��C�������ֱ�Ϊx��y����x+y=27��y-x=5�����x=11��y=16����BΪ��Ԫ�أ�CΪ��Ԫ�أ�0.9g D�ĵ��ʸ��������ᷴӦ������D3+��1.12L���������������D����Է�������Ϊz����
��3=
��2�����z=27����DΪ��Ԫ�أ�E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��E�γ�+1�����ӣ����Ӻ�����18�����ӣ���EΪ��Ԫ�أ���AΪ��Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�DΪ��Ԫ�أ�EΪ��Ԫ�أ�
��1���ɷ�����֪��AΪHԪ�أ�BΪNaԪ�أ�CΪSԪ�أ�DΪAlԪ�أ�EΪKԪ�أ��ʴ�Ϊ��H��Na��S��Al��K��
��2��B��D������������Ӧˮ����ΪNaOH��Al��OH��3����������������������Ӧ����ƫ��������ˮ���������Ӧ�����ӷ���ʽΪOH-��Al��OH��3=AlO2-��2H2O��
�ʴ�Ϊ��OH-��Al��OH��3=AlO2-��2H2O��
��3��K2S�����ӻ�����ɼ������������ӹ��ɣ��õ���ʽ��ʾK2S���γɹ���Ϊ
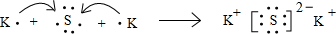
��
�ʴ�Ϊ��
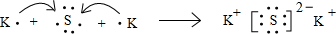
��
| 0.9 |
| z |
| 1.12L |
| 22.4L/mol |
��1���ɷ�����֪��AΪHԪ�أ�BΪNaԪ�أ�CΪSԪ�أ�DΪAlԪ�أ�EΪKԪ�أ��ʴ�Ϊ��H��Na��S��Al��K��
��2��B��D������������Ӧˮ����ΪNaOH��Al��OH��3����������������������Ӧ����ƫ��������ˮ���������Ӧ�����ӷ���ʽΪOH-��Al��OH��3=AlO2-��2H2O��
�ʴ�Ϊ��OH-��Al��OH��3=AlO2-��2H2O��
��3��K2S�����ӻ�����ɼ������������ӹ��ɣ��õ���ʽ��ʾK2S���γɹ���Ϊ
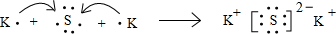
��
�ʴ�Ϊ��
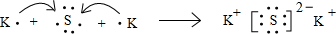
��

��ϰ��ϵ�д�
�����Ŀ
