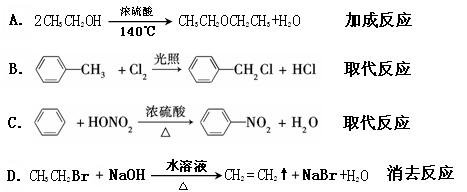
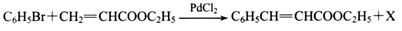��Ŀ����
��10�֣���һ������̼���⡢������Ԫ����ɵ��л���A��������3.2 g������ϵ�ȼ���ٽ����ɵ���������ͨ����ʢ��Ũ�����ϴ��ƿ�������ȵ�����ͭ���۱���ʯ��ˮ����ÿ��װ���еķ�Ӧ��������������ⶨ��������3.60g�����м���1.60g����������8.80g��A���������ܶ�Ϊ3.393g/L������ɱ�״�������ֲ�֪A������̼������Һ��Ӧ�ų�CO2���壬Ҳ��������Ʒ�Ӧ���������ɣ����ֱ�õ����������ͬ�����������ͬ��
��1��������л���ķ���ʽ_______________����д�����ܵĽṹ��ʽ_______________��
��2����ҵ�����������̺ϳ�A��

�ٵķ�Ӧ����Ϊ_____________������HOCH2CN�Ľṹʽ____________________��
��3��д��A��Ũ�������������������ã���д���������ʽ��
__________________________________________________________��
��1��������л���ķ���ʽ_______________����д�����ܵĽṹ��ʽ_______________��
��2����ҵ�����������̺ϳ�A��

�ٵķ�Ӧ����Ϊ_____________������HOCH2CN�Ľṹʽ____________________��
��3��д��A��Ũ�������������������ã���д���������ʽ��
__________________________________________________________��
(10��)
(1) C2H4O3��2�֣� HOCH2COOH��2�֣�
(2) �ӳɣ�1�֣�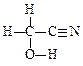 ��2�֣�
��2�֣�
(3)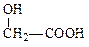 + CH3COOH
+ CH3COOH CH3COOCH2COOH + H2O��3�֣�
CH3COOCH2COOH + H2O��3�֣�
(1) C2H4O3��2�֣� HOCH2COOH��2�֣�
(2) �ӳɣ�1�֣�
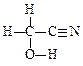 ��2�֣�
��2�֣�(3)
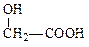 + CH3COOH
+ CH3COOH CH3COOCH2COOH + H2O��3�֣�
CH3COOCH2COOH + H2O��3�֣���

��ϰ��ϵ�д�
�����Ŀ
 _______________.
_______________. �л�������ʼҿ�ѧԺ����2010��ŵ������ѧ��������ķ�Ӧ�����ںտ˷�Ӧ���й��ж���ȷ����
�л�������ʼҿ�ѧԺ����2010��ŵ������ѧ��������ķ�Ӧ�����ںտ˷�Ӧ���й��ж���ȷ����