��Ŀ����
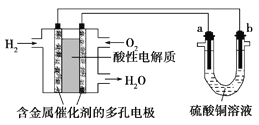
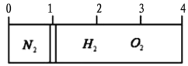
����Ŀ����ͼ��ʾ��CuSO45H2O����������480mL 0.20mol��L��1 CuSO4��Һ�ļ����ؼ�ʵ�鲽��Ͳ�������ͼ�ش��������⣺

��1��������ʵ�鲽��A��F��ʵ������Ⱥ�������� __________________�����ȡCuSO45H2O ���������Ϊ__________ g��
��2��д������480mL0.20mol��L��1CuSO4��Һ����Ҫ�õ��IJ������������ƣ��ձ�����Ͳ��_______________��
��3������Bͨ����Ϊת�ƣ���������NaOH��Һ����ˮ�ܽ�NaOH�����δ��ȴ�����¼�ת�ƣ�������Һ��Ũ�Ƚ�ƫ_____________��������������������������Aͨ����Ϊ____________��������ӿ̶��ߣ����Ƶ�Ũ�Ƚ�ƫ______����������������������
���𰸡�CBDFAE 25.0 500mol����ƿ�������� �� ���� ��
��������
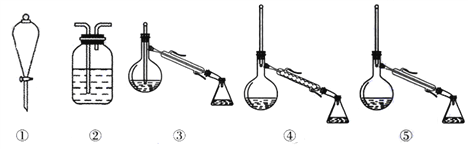
��1������һ�����ʵ���Ũ����Һ�IJ��裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������Ҫ�������������m=cVM��
��2������500mLһ�����ʵ���Ũ����Һ��Ҫ��������: ������ƽ��ҩ�ס��ձ�������������ͷ�ιܡ�500mL����ƿ��
��3��������Һ��Ũ������c=![]() �����жϡ�
�����жϡ�
��1������һ�����ʵ���Ũ����Һ�IJ��裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����A��F��ʵ������Ⱥ�������У�CBDFAE����Ҫ�������������m=cVM = 0.20mol/L��500mL��250g/mol =25.0g��
��2������500mLһ�����ʵ���Ũ����Һ����Ҫ�õ��IJ������������ƣ��ձ�����Ͳ��500mol����ƿ�����������ʴ�Ϊ��500mol����ƿ����������
��3������NaOH��Һ����ˮ�ܽ�NaOH�����δ��ȴ�����¼�ת�ƣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߡ�����Aͨ����Ϊ���ݣ�������ӿ̶��ߣ���Һ���ƫС�����Ƶ�Ũ�Ƚ�ƫ�ߡ��ʴ�Ϊ�������������ߡ�