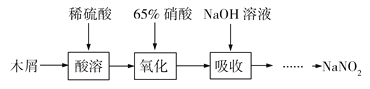
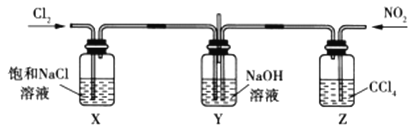
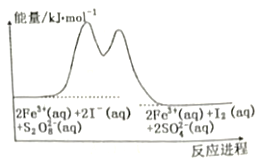
��Ŀ����
����Ŀ��ij���ͻ��������������Է�Һ�к������ֽ������ӣ�Fe3����Cu2������ѧС�����������ͼ��ʾ�ķ����Է�Һ���д������Ի��ս���������������
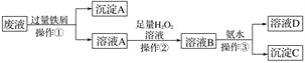
(1)�����ٵ�������________________�� ����A�к��еĽ���������________________��
(2)��ҺA�м���H2O2��Һ�����з�����Ӧ�����ӷ�Ӧʽ________________________________��������Ϊ�������ȱ��£��ڴ˹������¶Ȳ��˹��ߵ�ԭ����________________________________��
(3)������ҺB�к��еĽ��������ӵ�ʵ�鷽��Ϊ��ȡ������ҺB�ڽྻ��С�Թ��У�����______�Լ�����ҺѸ��ת��Ϊ________ɫ�����ɼ��顣
(4)�������з�����Ӧ�����ӷ���ʽΪ_________________________________________��
���𰸡����� ����ͭ 2Fe2����H2O2��2H��===2Fe3����2H2O H2O2�����ֽ� ���軯����Һ �� Fe3����3NH3��H2O===Fe(OH)3����3NH4+
��������
Fe3+��Cu2+����������Ӧ�����������Ӻ͵���ͭ��ͨ�������õ�����A����ҺA����������������AΪ����ͭ�Ļ�����ҺA���������ӣ��������Ӿ��л�ԭ�ԣ��ױ��������������ʼ�����������ܰ��������������������ӣ�����ҺB�к������ӣ������ӺͰ�ˮ��Ӧ�������������������ʳ���CΪ���������������õ�����ҺD����Ҫ����笠����ӣ���ˮͨ����������Fe3+��Cu2+�������ŷţ��ݴ˷������
(1)�����������Һ��ȡ���˵ķ����������ټ���Ĺ�������м���������Ļ�ѧ���ʱ�ͭ���ã���˿��ѽ���ͭ������Һ���û�������ͬʱFe3+����������������Fe2+������ڵڢٵõ��������ж����н���ͭ����������
�ʴ�Ϊ�����ˣ�����ͭ��
(2)��ҺA���������ӣ�����˫��ˮ����������ԭ��Ӧ����Ӧ�����ӷ�ӦʽΪ2Fe2����H2O2��2H��===2Fe3����2H2O��˫��ˮ�����ֽ⣬���Բ�����Ϊ�������ȱ��µ��¶Ȳ��˹��ߣ�
�ʴ�Ϊ��2Fe2����H2O2��2H��===2Fe3����2H2O��H2O2�����ֽ⣻
(3)B�к������ӣ������軯�������飬�����������軯�ط�Ӧ���ֺ�ɫ��Һ��
�ʴ�Ϊ�����軯����Һ���죻
(4)�����ӺͰ�ˮ��Ӧ������������������笠����ӣ���Ӧ�����ӷ���ʽΪFe3����3NH3��H2O===Fe(OH)3����3NH4+��
�ʴ�Ϊ��Fe3����3NH3��H2O===Fe(OH)3����3NH4+��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�