��Ŀ����
ij�л���X��C12H13O6Br������FeCl3��Һ����ɫ���䲿�ֽṹ��ʽ���£�
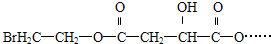
��֪X����������������ˮ��Һ�м��ȣ����Եõ�A��B��C�����л��
��1��C�ĺ˴Ź�������ֻ��һ�����շ壮
��2��������A�������ữ���Եõ�ƻ����E��E�Ľṹ��ʽΪ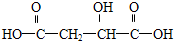 �Իش�
�Իش�
��1��B�����������ŵ�������
��2��C�Ľṹ��ʽΪ
 ��
��
��3��E���ܷ����ķ�Ӧ������
�ټӳɷ�Ӧ ����ȥ��Ӧ ��������Ӧ ��������Ӧ
��4��E��һ��ͬ���칹��F�������ص㣺lmol F���Ժ�3mol�����Ʒ�����Ӧ���ų�33.6LH2����״���£���lmolF���Ժ�����NaHCO3��Һ��Ӧ������lmolCO2��lmolF�����Է���������Ӧ������2molAg����д��F���ܵ�һ�ֽṹ��ʽ��

��5����һ��������������E���Է�Ӧ������Ԫ������д���˷�Ӧ�Ļ�ѧ����ʽ��

��6�����úϳɷ�Ӧ����ͼ��ʾ����1��3-����ϩΪ��Ҫ�л�ԭ�Ϻϳ�ƻ�����������ĺϳɷ�����ע����Ӧ��������
��ʾ�����л������д�ṹ��ʽ�����Լ���ѡ��
�ںϳɷ�Ӧ����ͼ��ʾ����ʾ�����£�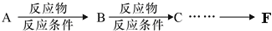
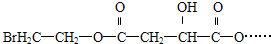
��֪X����������������ˮ��Һ�м��ȣ����Եõ�A��B��C�����л��
��1��C�ĺ˴Ź�������ֻ��һ�����շ壮
��2��������A�������ữ���Եõ�ƻ����E��E�Ľṹ��ʽΪ
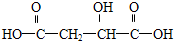 �Իش�
�Իش���1��B�����������ŵ�������
�ǻ�
�ǻ�
����2��C�Ľṹ��ʽΪ


��3��E���ܷ����ķ�Ӧ������
��
��
������ţ��ټӳɷ�Ӧ ����ȥ��Ӧ ��������Ӧ ��������Ӧ
��4��E��һ��ͬ���칹��F�������ص㣺lmol F���Ժ�3mol�����Ʒ�����Ӧ���ų�33.6LH2����״���£���lmolF���Ժ�����NaHCO3��Һ��Ӧ������lmolCO2��lmolF�����Է���������Ӧ������2molAg����д��F���ܵ�һ�ֽṹ��ʽ��


��5����һ��������������E���Է�Ӧ������Ԫ������д���˷�Ӧ�Ļ�ѧ����ʽ��


��6�����úϳɷ�Ӧ����ͼ��ʾ����1��3-����ϩΪ��Ҫ�л�ԭ�Ϻϳ�ƻ�����������ĺϳɷ�����ע����Ӧ��������
��ʾ�����л������д�ṹ��ʽ�����Լ���ѡ��
�ںϳɷ�Ӧ����ͼ��ʾ����ʾ�����£�
CH2=CHCH=CH2
CH2BrCH=CHCH2Br
CH2OHCH=CHCH2OH
CH2OHCH2CHClCH2OH
HOOCCH2CHClCOOH
| ��ˮ |
| NaOH |
| ˮ |
| һ�������� |
| ���� |
| һ�������� |
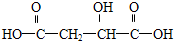
CH2=CHCH=CH2
CH2BrCH=CHCH2Br
CH2OHCH=CHCH2OH
CH2OHCH2CHClCH2OH
HOOCCH2CHClCOOH
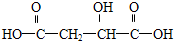
��| ��ˮ |
| NaOH |
| ˮ |
| һ�������� |
| ���� |
| һ�������� |
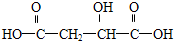
������X��FeCl3��Һ����ɫ��˵��X���зӵĽṹ����X����ɺͲ��ֽṹ��ʽ��֪��X�ɲ��ֽṹ��ʽ���ڱ�������ɣ���ΪC�ĺ˴Ź�������ֻ��һ�����շ壬����X�б����ϵ�����ȡ�������ڶ�λ��XΪ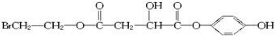 ������E�Ľṹ��֪AΪ
������E�Ľṹ��֪AΪ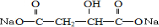 ��BΪCH2OHCH2OH��CΪ
��BΪCH2OHCH2OH��CΪ ������л���Ľṹ�����ʽ����⣮
������л���Ľṹ�����ʽ����⣮
 ������E�Ľṹ��֪AΪ
������E�Ľṹ��֪AΪ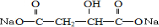 ��BΪCH2OHCH2OH��CΪ
��BΪCH2OHCH2OH��CΪ ������л���Ľṹ�����ʽ����⣮
������л���Ľṹ�����ʽ����⣮����⣺X��FeCl3��Һ����ɫ��˵��X���зӵĽṹ����X����ɺͲ��ֽṹ��ʽ��֪��X�ɲ��ֽṹ��ʽ���ڱ�������ɣ���ΪC�ĺ˴Ź�������ֻ��һ�����շ壬����X�б����ϵ�����ȡ�������ڶ�λ��XΪ ������E�Ľṹ��֪AΪ
������E�Ľṹ��֪AΪ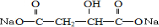 ��BΪCH2OHCH2OH��CΪ
��BΪCH2OHCH2OH��CΪ ��
��
�� l ��BΪCH2OHCH2OH�����еĹ�����Ϊ�ǻ����ʴ�Ϊ���ǻ���
��2�������Ϸ�����֪BΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��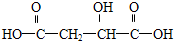 �к����Ȼ����ɷ���������Ӧ������-OH���ɷ���������Ӧ����ȥ��Ӧ����C=C�����ܷ����ӳɷ�Ӧ���ʴ�Ϊ���٣�
�к����Ȼ����ɷ���������Ӧ������-OH���ɷ���������Ӧ����ȥ��Ӧ����C=C�����ܷ����ӳɷ�Ӧ���ʴ�Ϊ���٣�
��4��EΪ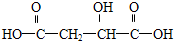 ����Ӧ��ͬ���칹��F��lmolF���Ժ�3mol�����Ʒ�����Ӧ���ų�33.6LH2����״���£���˵�������к���3������Na��Ӧ��-OH��-COOH��lmolF���Ժ�����NaHCO3��Һ��Ӧ������lmolCO2��˵������1��-COOH��lmolF�����Է���������Ӧ������2molAg��˵������1��-CHO����Ӧ����2��-OH�����ܵĽṹΪ
����Ӧ��ͬ���칹��F��lmolF���Ժ�3mol�����Ʒ�����Ӧ���ų�33.6LH2����״���£���˵�������к���3������Na��Ӧ��-OH��-COOH��lmolF���Ժ�����NaHCO3��Һ��Ӧ������lmolCO2��˵������1��-COOH��lmolF�����Է���������Ӧ������2molAg��˵������1��-CHO����Ӧ����2��-OH�����ܵĽṹΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��5����һ��������������E���Է�Ӧ������Ԫ��������Ӧ�ķ���ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6����1��3-����ϩΪ��Ҫ�л�ԭ�Ϻϳ�ƻ���ᣬӦ�����巢��1��4�ӳ�����CH2BrCH=CHCH2Br��Ȼ��ˮ������CH2OHCH=CHCH2OH����HCl�����ӳɷ�Ӧ����CH2OHCH2CHClCH2OH����������HOOCCH2CHClCOOH�����ˮ�������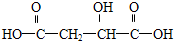 ����Ӧ������ΪCH2=CHCH=CH2
����Ӧ������ΪCH2=CHCH=CH2
CH2BrCH=CHCH2Br
CH2OHCH=CHCH2OH
CH2OHCH2CHClCH2OH
HOOCCH2CHClCOOH
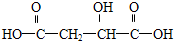 ��
��
�ʴ�Ϊ��CH2=CHCH=CH2
CH2BrCH=CHCH2Br
CH2OHCH=CHCH2OH
CH2OHCH2CHClCH2OH
HOOCCH2CHClCOOH
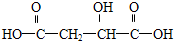 ��
��
 ������E�Ľṹ��֪AΪ
������E�Ľṹ��֪AΪ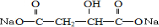 ��BΪCH2OHCH2OH��CΪ
��BΪCH2OHCH2OH��CΪ ��
���� l ��BΪCH2OHCH2OH�����еĹ�����Ϊ�ǻ����ʴ�Ϊ���ǻ���
��2�������Ϸ�����֪BΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����3��
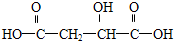 �к����Ȼ����ɷ���������Ӧ������-OH���ɷ���������Ӧ����ȥ��Ӧ����C=C�����ܷ����ӳɷ�Ӧ���ʴ�Ϊ���٣�
�к����Ȼ����ɷ���������Ӧ������-OH���ɷ���������Ӧ����ȥ��Ӧ����C=C�����ܷ����ӳɷ�Ӧ���ʴ�Ϊ���٣���4��EΪ
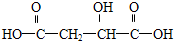 ����Ӧ��ͬ���칹��F��lmolF���Ժ�3mol�����Ʒ�����Ӧ���ų�33.6LH2����״���£���˵�������к���3������Na��Ӧ��-OH��-COOH��lmolF���Ժ�����NaHCO3��Һ��Ӧ������lmolCO2��˵������1��-COOH��lmolF�����Է���������Ӧ������2molAg��˵������1��-CHO����Ӧ����2��-OH�����ܵĽṹΪ
����Ӧ��ͬ���칹��F��lmolF���Ժ�3mol�����Ʒ�����Ӧ���ų�33.6LH2����״���£���˵�������к���3������Na��Ӧ��-OH��-COOH��lmolF���Ժ�����NaHCO3��Һ��Ӧ������lmolCO2��˵������1��-COOH��lmolF�����Է���������Ӧ������2molAg��˵������1��-CHO����Ӧ����2��-OH�����ܵĽṹΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����5����һ��������������E���Է�Ӧ������Ԫ��������Ӧ�ķ���ʽΪ
 ��
���ʴ�Ϊ��
 ��
����6����1��3-����ϩΪ��Ҫ�л�ԭ�Ϻϳ�ƻ���ᣬӦ�����巢��1��4�ӳ�����CH2BrCH=CHCH2Br��Ȼ��ˮ������CH2OHCH=CHCH2OH����HCl�����ӳɷ�Ӧ����CH2OHCH2CHClCH2OH����������HOOCCH2CHClCOOH�����ˮ�������
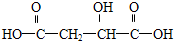 ����Ӧ������ΪCH2=CHCH=CH2
����Ӧ������ΪCH2=CHCH=CH2| ��ˮ |
| NaOH |
| ˮ |
| һ�������� |
| ���� |
| һ�������� |
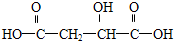 ��
���ʴ�Ϊ��CH2=CHCH=CH2
| ��ˮ |
| NaOH |
| ˮ |
| һ�������� |
| ���� |
| һ�������� |
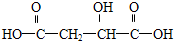 ��
�����������⿼���л�����ƶϣ���Ŀ�ѶȽϴ���ע�����X�����ʽ��E�Ľṹ��ȷ�ƶ�X�Ľṹ���ƶ��������ʵĽṹ��ʽΪ������Ĺؼ����״���Ϊ��6����ע��������ʵĽṹ����������Ʊ����̣�

��ϰ��ϵ�д�
�����Ŀ

 Z+H2O��д���÷�Ӧ�Ļ�ѧ����ʽ
Z+H2O��д���÷�Ӧ�Ļ�ѧ����ʽ

 �������������غ㶨�ɣ����жϳ���һ����Ӧ����Ϊ
�������������غ㶨�ɣ����жϳ���һ����Ӧ����Ϊ ij�л���X�ļ���ʽΪ��
ij�л���X�ļ���ʽΪ��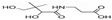 �������л���X��˵����ȷ���ǣ�������
�������л���X��˵����ȷ���ǣ�������