��Ŀ����
����С����ע��������Ӧ������ͭ��ϡHNO3��Ӧ��ʵ������˴�����ƣ�����ʵ���������й����⣨�ٶ�ʵ���ڱ�״���½��У�������N2��O2�������Ϊ4��1����
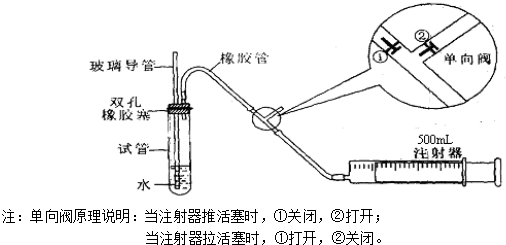
��1����ͼ��һ֧���Ϊ50 mL��ע��������������Ͳ����������ã����γ���ͷ������48 mgͭƬ��Ȼ���ڰ���ͷ�����������ܡ�
��2���ȳ�ȡ10 mol��L-1��ϡHNO3��������������Һ���У�Ŀ����______________________��
��3���ٳ�ȡ10 mL 6 mol��L-1��ϡHNO3���ѵ��ɼм����������ϣ���Ӧ��ʼʱ���������ӿ졣Ԥ����ע�����пɹ۲쵽��Щ����������д����
��4����Ӧ��Ϻ�ֹˮ�У���������������36.2 mL�����кõ��ɼУ��۲쵽___________����ҡע�������£�����Ƭ���ֹ۲쵽_________________________���ظ����ϲ������Σ�����Ͳ�������NOΪֹ����Ͳ����Һ��c��![]() ��Ϊ___________��c��H+��Ϊ___________��
��Ϊ___________��c��H+��Ϊ___________��
��5����ʵ�����������ŵ���_________________________________________________��
������2����Ϊ���ž�ע�����ڲ���������������
��3����ͭ��ϡ���ᷴӦ֪����ͭƬ�����������ݳ�����Ͳ�ھۼ���ɫ���壻����Һ����ɫ��Ϊ��ɫ������ͭ����������ʵ����ж������������ͭƬȫ���ܽ⣬���ͨ������48 mgͭƬ��ȫ��Ӧ����������ˮ��NO 11.2 mL���ܻ����Զ��ƶ����̶�11.2 mL����
��4��NO������Ŀ�������Ϊ����ɫ��NO2������ɫ��NO2��ˮ��Ӧ�ֱ�Ϊ��ɫ����֪3O2+4NO+2H2O![]() 4HNO3���������������7 mL��15 mL�����е�3 mL O2��4 mL NO����������36.2 mL�������Ƶ�29.2 mL�����ӽ����,
4HNO3���������������7 mL��15 mL�����е�3 mL O2��4 mL NO����������36.2 mL�������Ƶ�29.2 mL�����ӽ����,![]() Ũ��Ӧ�غ�,��Ϊ6 mol��L-1�����ܷ�Ӧ:2Cu+O2+4H+
Ũ��Ӧ�غ�,��Ϊ6 mol��L-1�����ܷ�Ӧ:2Cu+O2+4H+![]() 2Cu2++2H2O�ɼ����H+Ũ��Ϊ5.85 mol��L-1��
2Cu2++2H2O�ɼ����H+Ũ��Ϊ5.85 mol��L-1��
��5������Ⱦ��

 ͭ��Ũ���ᷴӦ��ͭ��ϡ���ᷴӦ�����ﲻͬ��ʵ������Ҳ��ͬ��ij����С��Ϊ��֤�����۲쵽ͭ��ϡ���ᷴӦ�IJ���ΪNO���������ͼ��ʾ��ʵ��װ�ã�����������ǵ�˼·��ѡ������ҩƷ����ɸ�ʵ�飬������ʵ�鲽�裮ҩƷ��ϡ���ᡢ���ᡢZn����CaCO3����
ͭ��Ũ���ᷴӦ��ͭ��ϡ���ᷴӦ�����ﲻͬ��ʵ������Ҳ��ͬ��ij����С��Ϊ��֤�����۲쵽ͭ��ϡ���ᷴӦ�IJ���ΪNO���������ͼ��ʾ��ʵ��װ�ã�����������ǵ�˼·��ѡ������ҩƷ����ɸ�ʵ�飬������ʵ�鲽�裮ҩƷ��ϡ���ᡢ���ᡢZn����CaCO3����