��Ŀ����
ij��ѧС�������ۼ���ϡ���Ũ�����ʵ��ʱ����������±����밴�ո����ļ�����ʽ����д���������ֲ�ͬ�ļ�����
��1���ס�����ͬѧ������������װ�ü�������ԣ�
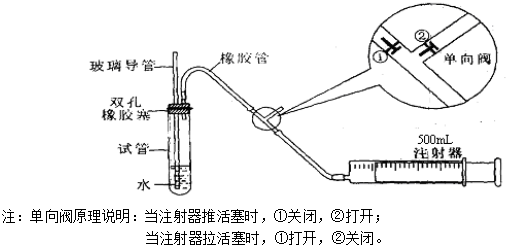
��ͬѧ��������ס�Թܣ������Թܣ����۲쵼�����Ƿ���Һ�������������Ƿ��ܣ� �����ܻ��ܣ���
��ͬѧ���ȴӲ����������Թ���ע��ˮ���۲쵼����Һ�����Թ���Һ���Ƿ��γɸ߶Ȳ����һ��ʱ��۲�Һ����Ƿ�仯�ķ������Ƿ��ܣ� �����ܻ��ܣ���
���������ַ��������ܣ���˼���·������������пհף����������У��������пհף��������Թ���װ������ˮ����֤�������ܵ��¶˽�û��ˮ�У���Ȼ������������� ������ ����ʵ������֤����װ�õ����������ã�
��2�����Թ��м���0.00127%�ĵ�ˮ10g��������������ˮϡ�ͺ��ټ���2��3�ε�����Һ�����ó���ҺA���ⶨָ���ص������SO2�ĺ���ʱ������ע�����Ļ��������������Թ��з����Ļ�ѧ��Ӧ����ʽΪSO2+I2+2H2O=H2SO4+2HI��A��Һ�� ɫ��Ϊ ʱ������Ӧǡ����ȫ���У���ʱӦֹͣ������
��3���ҹ����������������ж�ÿ�ο��������ⶨ��SO2�����Ũ����ֵ��mg/m3����һ������0.15����������0.50����������0.70���ÿ���С��ֳɵ�һ�С��͵ڶ��С�飬ʹ����ͬ��ʵ��װ�ú���ҺA����ͬһ�ص㡢ͬʱ����������SO2�ĺ���������Ӧǡ����ȫ���У���¼����ʱ��ʹ������£��ٶ�ÿ�γ���500mL�����뽫�±���д����������ʱ����2λ��Ч���֣���
����ʵ������У��軺���鶯������Ŀ���� �������ٳ�������ⶨ�Ľ���� ����ƫ��ƫС����Ӱ�죩��
���жϸõص�Ŀ�����SO2�������� �������֣������� �����һ���ڶ�����С��IJⶨ���ȷ����һС��ʵ���������ϴ�ƫ���ԭ���� ������С������ҩƷ��װ�þ������⣩��
| ���� | ���� | ���� |
| �� | �ֱ���뵽ʢˮ���Թ��� | ������ΪŨ���� |
| �� | ||
| �� | ||
| �� |

��ͬѧ��������ס�Թܣ������Թܣ����۲쵼�����Ƿ���Һ�������������Ƿ��ܣ�
��ͬѧ���ȴӲ����������Թ���ע��ˮ���۲쵼����Һ�����Թ���Һ���Ƿ��γɸ߶Ȳ����һ��ʱ��۲�Һ����Ƿ�仯�ķ������Ƿ��ܣ�
���������ַ��������ܣ���˼���·������������пհף����������У��������пհף��������Թ���װ������ˮ����֤�������ܵ��¶˽�û��ˮ�У���Ȼ�������������
��2�����Թ��м���0.00127%�ĵ�ˮ10g��������������ˮϡ�ͺ��ټ���2��3�ε�����Һ�����ó���ҺA���ⶨָ���ص������SO2�ĺ���ʱ������ע�����Ļ��������������Թ��з����Ļ�ѧ��Ӧ����ʽΪSO2+I2+2H2O=H2SO4+2HI��A��Һ��
��3���ҹ����������������ж�ÿ�ο��������ⶨ��SO2�����Ũ����ֵ��mg/m3����һ������0.15����������0.50����������0.70���ÿ���С��ֳɵ�һ�С��͵ڶ��С�飬ʹ����ͬ��ʵ��װ�ú���ҺA����ͬһ�ص㡢ͬʱ����������SO2�ĺ���������Ӧǡ����ȫ���У���¼����ʱ��ʹ������£��ٶ�ÿ�γ���500mL�����뽫�±���д����������ʱ����2λ��Ч���֣���
| ���� | ��һС�� | �ڶ�С�� |
| ����ʱ�� | 20���� | 21���� |
| �������� | 100 | 130 |
| SO2����mg/m3 |
���жϸõص�Ŀ�����SO2��������
����������Ũ�����ϡ���ᣬ�ɸ��ݶ������ʵ���ͬ��Ũ�������ǿ�����ԡ���ˮ�Ժ���ˮ�ԣ�
��1��Ū��װ�������Եļ������Ҫ��֤װ�����γ�һ���ܱյ���ϵ�������ܱ���ϵ�ڵ�ѹǿ�ı仯�����ڵ�ע�����ƻ���ʱ���ٹرգ��ڴ���ע����������ʱ���ٴ��ڹرգ���˼���װ�õ�������ʱ��Ϊʹ�γ�һ���ܱյĿռ䣬Ӧ��ע�����Ļ�����ʹװ���ڵ�ѹǿ��С���Ӷ��ڽ�û��ˮ�еIJ������ܿڻῴ��������ð����
��2������SO2+I2+2H2O=H2SO4+2HI�������ʵ�ǡ����ȫ��Ӧʱ�����������ɵ���ɫ�ͻ���ʧ��
��3����Ϸ�Ӧ�ķ���ʽ�����ݲμӷ�Ӧ�ĵ�����������������������������Ӷ�ȷ���˵ؿ����ж�������ĺ�����
��1��Ū��װ�������Եļ������Ҫ��֤װ�����γ�һ���ܱյ���ϵ�������ܱ���ϵ�ڵ�ѹǿ�ı仯�����ڵ�ע�����ƻ���ʱ���ٹرգ��ڴ���ע����������ʱ���ٴ��ڹرգ���˼���װ�õ�������ʱ��Ϊʹ�γ�һ���ܱյĿռ䣬Ӧ��ע�����Ļ�����ʹװ���ڵ�ѹǿ��С���Ӷ��ڽ�û��ˮ�еIJ������ܿڻῴ��������ð����
��2������SO2+I2+2H2O=H2SO4+2HI�������ʵ�ǡ����ȫ��Ӧʱ�����������ɵ���ɫ�ͻ���ʧ��
��3����Ϸ�Ӧ�ķ���ʽ�����ݲμӷ�Ӧ�ĵ�����������������������������Ӷ�ȷ���˵ؿ����ж�������ĺ�����
����⣺Ũ��������ˮ�ų��������ȣ�������ˮ�ԣ��ɼ��뵽CuSO4?5H2O�У�ʹ����ʧȥ�ᾧˮ����ɰ�ɫ��Ũ����е�ϸߣ�������ͬ�����¼��ȣ��ȷ��ڵ�Ϊϡ�����ֱ������ͬ������ᣬ���������Ũ���ᣬҲ�ɼ���ͭ�����ȣ�ͭ�ܽ��ΪŨ����ȣ�
�ʴ�Ϊ��
��1�����ڲ��������������ͬ��û�γ��ܱյ���ϵ��ֱ��������ķ������ܼ��������ԣ�����ͬѧ��Ȼ��������עˮ��û��ʹ��ѹǿ�����仯���������ͬѧ��û�������װ�õ��������Ƿ���ã���Ҫ�����װ�õ������ԣ��������ⵥ�����ã�����ԭ��˵������ע�����ƻ���ʱ���ٹرգ��ڴ���ע����������ʱ���ٴ��ڹرգ����Ӧ������ע�����Ļ����������Թ���ѹǿ��С����������û��ˮ�еIJ����ܿڴ�������ð����˵�����������ã�
�ʴ�Ϊ�����ܣ����ܣ�������������ע�����Ļ�������û��ˮ�еIJ������ܿ�������ð����
��2���ӻ�ѧ����ʽSO2+I2+2H2O=H2SO4+2HI���ѿ���������Ӧ��ǡ����ȫ��Ӧʱ����Һ�в����е��ʵ⣬��Һ����ɫ�ͻ���ʧ���ʴ�Ϊ��������ɫ��
��3��������ȡ�õ�ˮ��һ���ģ��������ʵ���в��뷴Ӧ�Ķ������������һ���࣬Ϊ��ʹ��������������ȫ�����գ���˳�������ʱҪ�����ؽ��У����ݻ�ѧ����ʽ���㣬����뷴Ӧ�Ķ������������ΪX��
SO2 +I2 +2H2O=H2SO4 +2HI
64 254
X 10g��0.00127%
=
X=
=0.000032g=0.032mg��
��һ�飺
=0.64mg/m3
�ڶ��飺
=0.49mg/m3
�ʴ�Ϊ��0.64mg/m3��0.49mg/m3��
�����������ݿ�֪����ʵ������У��軺���鶯������Ŀ�����ö�����������ַ�Ӧ����ʹ�����ж�����������գ�������ᵼ�²ⶨ���ƫС��
�ʴ�Ϊ���ö�����������ַ�Ӧ����ʹ�����ж�����������գ���ƫС��
�ڸõص�Ŀ�����SO2����Ϊ0.64mg/m3������0.50��ӦΪ����������һС��IJⶨ�����Ϊȷ����ڶ�С��������ʹ��죬��ɿ�����SO2���ˮδ��ַ�Ӧ�������ϴ���
�ʴ�Ϊ����������һ���������ʹ��죬��ɿ�����SO2���ˮδ��ַ�Ӧ�������ϴ���
�ʴ�Ϊ��
| �ֱ�ε������ | �����ΪŨ���� |
| �ֱ������Ƭ | �������ܽ����ϡ���� |
| �ֱ�ӵ�CuSO4?5H2O�� | �����ΪŨ���� |
| �ֱ�����ͬ�����¼��� | �ȷ�������ϡ���� |
| �ֱ������ͬ������� | ���������Ũ���� |
�ʴ�Ϊ�����ܣ����ܣ�������������ע�����Ļ�������û��ˮ�еIJ������ܿ�������ð����
��2���ӻ�ѧ����ʽSO2+I2+2H2O=H2SO4+2HI���ѿ���������Ӧ��ǡ����ȫ��Ӧʱ����Һ�в����е��ʵ⣬��Һ����ɫ�ͻ���ʧ���ʴ�Ϊ��������ɫ��
��3��������ȡ�õ�ˮ��һ���ģ��������ʵ���в��뷴Ӧ�Ķ������������һ���࣬Ϊ��ʹ��������������ȫ�����գ���˳�������ʱҪ�����ؽ��У����ݻ�ѧ����ʽ���㣬����뷴Ӧ�Ķ������������ΪX��
SO2 +I2 +2H2O=H2SO4 +2HI
64 254
X 10g��0.00127%
| 64 |
| 254 |
| X |
| 10g��0.00127% |
X=
| 64��10g��0.00127% |
| 254 |
��һ�飺
| 0.032mg |
| 500��100��10-6m3 |
�ڶ��飺
| 0.032mg |
| 500��130��10-6m3 |
�ʴ�Ϊ��0.64mg/m3��0.49mg/m3��
�����������ݿ�֪����ʵ������У��軺���鶯������Ŀ�����ö�����������ַ�Ӧ����ʹ�����ж�����������գ�������ᵼ�²ⶨ���ƫС��
�ʴ�Ϊ���ö�����������ַ�Ӧ����ʹ�����ж�����������գ���ƫС��
�ڸõص�Ŀ�����SO2����Ϊ0.64mg/m3������0.50��ӦΪ����������һС��IJⶨ�����Ϊȷ����ڶ�С��������ʹ��죬��ɿ�����SO2���ˮδ��ַ�Ӧ�������ϴ���
�ʴ�Ϊ����������һ���������ʹ��죬��ɿ�����SO2���ˮδ��ַ�Ӧ�������ϴ���
�����������ۺϿ������ʵĺ����ⶨ��̽����������ѧ���ķ�������������������ʵ�������Ŀ��飬Ϊ�߿��������ͣ�ע�����ʵ��ԭ����������ע�������Ϸ�Ӧ�Ļ�ѧ����ʽ�����⣮

��ϰ��ϵ�д�
�����Ŀ
ij��ѧС�������ۼ���ϡ�����Ũ�����ʵ��ʱ��������·�����
| �١������� | �ᡡ������ | |
| �� | ��� | �����ΪŨ���� |
| �� | ���������Ƭ | �����̼�����ζ��ΪŨ���� |
| �� | �ֱ���뵽ʢˮ���Թ��� | �Ŵ�������ΪŨ���� |
| �� | ���Լ�ƿ�� | ð������ΪŨ���� |
| �� | �ӵ�CuSO4?5H2O ������ | ʹ��������ΪŨ���� |
��2������һ�����۵������ܳ�Ϊ���е���______��������______��
��3����ȫ�������______����Ϊ______��
��4����������ȷ�����⣬���������һ�ּ�����
| �١������� | �ᡡ������ | |
| ______ | ______ |
ij��ѧС�������ۼ���ϡ�����Ũ�����ʵ��ʱ��������·�����
��1��������______��
��2������һ�����۵������ܳ�Ϊ���е���______��������______��
��3����ȫ�������______����Ϊ______��
��4����������ȷ�����⣬���������һ�ּ�����
| �� �� | �� �� | |
| �� | ��� | �����ΪŨ���� |
| �� | ���������Ƭ | �����̼�����ζ��ΪŨ���� |
| �� | �ֱ���뵽ʢˮ���Թ��� | �Ŵ�������ΪŨ���� |
| �� | ���Լ�ƿ�� | ð������ΪŨ���� |
| �� | �ӵ�CuSO4?5H2O ������ | ʹ��������ΪŨ���� |
��2������һ�����۵������ܳ�Ϊ���е���______��������______��
��3����ȫ�������______����Ϊ______��
��4����������ȷ�����⣬���������һ�ּ�����
| �� �� | �� �� | |
| ______ | ______ |