��Ŀ����
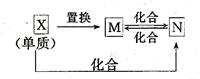
(15��)A��B��C��Ϊ��ѧ��ѧ�����ĵ��ʣ�����һ���ǽ�����ͨ��״���£�AΪ���壬BΪ����ɫ���壬CΪ���壬�����Ϊ�����X��һ�ֳ��õľ��лӷ��Ե�����ǿ�ᣬEΪ��ɫ���壬HΪ��ɫҺ�壬����֮���ת����ϵ��ͼ������ijЩ��Ӧ�����Ͳ�������ȥ����

��1��д����A�Ļ�ѧʽ ��F�Ļ�ѧʽ ��
��2���ڷ�Ӧ�١����У�������������ԭ��Ӧ���� ������ţ���
��3����ɣ�E��X��Ӧ�����ӷ���ʽ�� ��
��4�����H��ij�ֻ����������C�Ļ�ѧ����ʽ
 ��ÿ����0.15molC����ת�� mol��
��ÿ����0.15molC����ת�� mol��
��5��B�����������������а��̼���һ�����嵥�ʲ������÷�Ӧ�Ļ�ѧ����ʽΪ��
��

��1��д����A�Ļ�ѧʽ ��F�Ļ�ѧʽ ��
��2���ڷ�Ӧ�١����У�������������ԭ��Ӧ���� ������ţ���
��3����ɣ�E��X��Ӧ�����ӷ���ʽ�� ��
��4�����H��ij�ֻ����������C�Ļ�ѧ����ʽ

 ��ÿ����0.15molC����ת�� mol��
��ÿ����0.15molC����ת�� mol����5��B�����������������а��̼���һ�����嵥�ʲ������÷�Ӧ�Ļ�ѧ����ʽΪ��
��
(15��)��1��Fe(1��) KSCN����NH4SCN��(1��) ��2���ۢ�(2��)
��3��Fe3O4+8H+=Fe2++2Fe3++4H2O(3��)
��4��2Na2O2+2H2O=4NaOH+O2��(3��) 0.3(2��) (5)3Cl2+8NH3=6NH4Cl+N2(3��)
��3��Fe3O4+8H+=Fe2++2Fe3++4H2O(3��)
��4��2Na2O2+2H2O=4NaOH+O2��(3��) 0.3(2��) (5)3Cl2+8NH3=6NH4Cl+N2(3��)
��

��ϰ��ϵ�д�
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
�����Ŀ


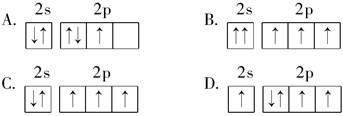

 Ϊ
Ϊ
 D���ȼ�
D���ȼ�